Dysfunction
Grade 0
Grade 1
Grade 2
Grade 3
Pulmonary PaO2/FiO2
>250
250–200
200–100
<100
Renal creatinine (mg/dl)
<1.8
1.8–2.5
2.5–5.0
>5
Hepatic total bilirubin (mgdl/l)
<2
2–4
4–8
>8
Cardiac
No inotropes
Only 1 inotrope at small dose
Any inotrope at moderate dose or >1 agent at small dose
Any inotrope at large dose or >2 agent at moderate doses
Table 14.2
SOFA score
Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
Respiratory PaO2/FiO2 | >400 | <400 | <300 | <20 with respiratory support | <100 with respiratory support |
Coagulation platelets (×103/mm3) | >150 | <150 | <100 | <50 | <20 |
Liver – bilirubin (mg/dl) | <1.2 | 1.2–1.9 | 2.0–5.9 | 6–11.9 | >12 |
Cardiovascular (vasopressor in mcg/kg/min) | No hypotension | MAP <70 mmHg | Dopamine < = 5 or dobutamine (any dose) | dopamine > 5 OR epi < = 0.1 OR nor epi < = 0.1 | dop > 15 OR epi > 0.1 OR nor epi > 0.1 |
Renal – creatinine (mg/dl) | <1.2 | 1.2–1.9 | 2.0–3.4 | 3.5–4.9 | >5 |
CNS – GCS | 15 | 13–14 | 10–12 | 6–9 | <6 |
We can identify two types of MOF depending on the time of onset:
14.3 Epidemiology
The mortality of MOF in traumatic patients continues to decline in the course of the years, thanks to the progress made by treatment strategy and new approaches in intensive care unit. In the early 1980s the mortality rate was about 60–100 % [8]; today patients who get postinjury MOF have a mortality rate of 27–100 % depending on the number of organs involved [4].
The incidence of the syndrome instead shows an increase, from the first studies of the 1980s to those of the 1990s, from about 7 to 15 % and remains the same even today [6, 8–10].
Probably this is due to the better understanding of the syndrome and consequently greater ability to recognize and differentiate it.
The most important determinants of MOF’s incidence and mortality are factors such as the type and gravity of trauma; different sex, age, and comorbidities; and number of patients who required massive blood transfusion.
The incidence and mortality of single organ failures are also decreased significantly over the years from 22 to 7 % and from 30 to 11 %, respectively [6, 8–10]. The organs affected, however, remain the same, even with the same frequency of interest: lung failure, which usually precedes the cardiac dysfunction of 0.6 days, followed by the liver (4.8 days) and finally by the kidney (5.5 days) [11].
The MOF syndrome is difficult to handle even by the most experienced and remains a major cause of ICU resource use and late mortality after injury.
14.4 Etiology
As previously mentioned, the etiology of the MOF is varied and complex and depends on both the factors related to the patient – age, sex, BMI, comorbidities, and genetic predisposition – and the characteristics of different types of trauma: severity, ISS, contamination of wounds, and time from injury to treatment.
According to the “second-hit theory,” the concept that underlies the onset of the MOF syndrome is the existence of an event that acts as first injury, the “first hit,” determinating a proinflammatory reaction. It depends on:
The local damage to tissues and systemic inflammation
The systemic hypoperfusion following hypotension and oxygenation impairment
The reperfusion injury which follows the initial successful management
Subsequently an event, such as lung infections, prolonged shock, excessive volume of crystalloid infusions, units of red blood cells, surgery, mechanical ventilation, fat embolism, compartment syndrome, and sepsis, acts as a “second hit,” resulting in an uncontrolled inflammation, usually starting during the “vulnerable window” (neutrophilia that occurs early after the traumatic event) [4, 12] and developing the early SIRS in organ failure (Fig. 14.1).
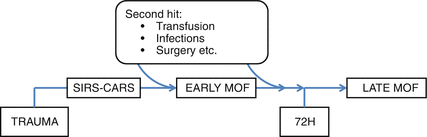

Fig. 14.1
Timing of impending MOF
14.5 Pathophysiology
After the trauma, many proinflammatory cytokines, such as IL-1b, IL-6, TNFa, IL-8, IL-12, IL-18, G-CSF, GM-CSF, are immediately released into the circulation that will activate PMNs but also a variety of anti-inflammatory cytokines, for example, IL-1Ra, IL-4, IL-10, IL-11 and IL-13. Usually in homeostasis conditions both sides are balanced and the result is equilibrium. The predominance of the proinflammatory phase leads to SIRS, while the prevalence or lack of control of the inflammatory phase leads to immunosuppression. As mentioned before, a second hit during the CARS or the “vulnerable window,” such as an infection, a massive blood transfusion, surgery, respiratory distress with hypoxia, repeated cardiovascular instability, acidosis, and missed injuries, triggers the MOF [13–15].
Some recent studies [16, 17] have shown that in polytrauma patient there are elevated levels of cytokines as TNFs and IL-6 proportionally to the severity of the trauma, confirming the role of this interleukin as a marker of the severity of trauma and as a useful parameter to try to predict the onset of MOF.
TNF-alpha is a cytokine involved in inflammation and stimulates the acute phase reaction; its primary role is in the regulation of immune response. It stimulates the production of NO and activates the cyclooxygenase, promoting the production of thromboxane, prostaglandins, and PAF, unbalancing the endothelial system on behalf the procoagulant factors. TNF-alpha also increases capillary permeability, promoting the migration of neutrophils into the tissue.
The IL-6 is another important cytokine in the genesis of SIRS in trauma; it regulates inflammation; generates C-reactive protein, procalcitonin and fibrinogen; and activates lympho-cytes and NK cells. In the genesis of MOF in injured patients, the IL-6 confirms its dual role as both proinflammatory and anti-inflammatory.
The IL-10 is one of most important anti-inflammatory cytokine; its role is exactly the opposite that of the abovementioned cytokines, for example, inhibits the production of monocytes/macrophages and reduces the proinflammatory mediators.
The complement system plays a key role in the genesis of inflammation after trauma. Sharma et al., Hecke et al., and Kapur et al. have shown that the plasma levels of proinflammatory peptides C3 and C3a increase immediately after trauma [18–20]. The complement cascade causes the formation of pores in cell membranes, resulting in lysis and the production of oxygen free radicals and arachidonic acid metabolites.
In the pathogenesis of MOF are also involved the ischemia/reperfusion injury pathway and the “gut’s role.”
Ischemia reduces the reserves of ATP, increasing the levels of hypoxanthine and raising intracellular levels of Na+ and altering those of Ca2+. During the secondary reperfusion, the oxygen reacts with hypoxanthine, producing H2O2 and the hydroxyl radical °OH−; these reactive oxygen species (ROS, oxygen radicals) contribute to the cell membrane peroxidation, causing diffuse tissue damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


