Fig. 7.1
The role of imaging in cardiac sarcoidosis
1.
Diagnosis – Establish the presence or absence of cardiac involvement.
2.
Prognosis – Identify patients who have a high (or low) risk of adverse events.
3.
Identify patients who are most likely to benefit from immunosuppressive therapies.
4.
Follow response to therapy.
With respect to the above goals, there is often a complementary value when combining data from various imaging modalities. For instance, information from both cardiac PET and MRI can be used to more confidently assess the likelihood of cardiac involvement. Each of these tests can image different aspects of cardiac disease making the combined findings from these two techniques particularly valuable. Such a hybrid approach may be especially important when the findings of any one of these tests provides inconclusive results, or when the diagnosis is challenging to establish (e.g. isolated cardiac involvement).
This chapter will discuss the role and importance of cardiac imaging in evaluating patients with known or suspected cardiac sarcoidosis. The chapter will discuss how to select among the various existing testing options as well as how to use the results provided by these tests in patient management.
When Is Advanced Multimodality Imaging Needed in a Patient with Suspected Cardiac Sarcoidosis?
The incidence of systemic sarcoidosis is increasing, and the death rate from sarcoidosis has increased 51 % from 1988 to 2007 [1]. The majority of these potentially preventable deaths in patients with sarcoidosis are from cardiac causes, but a significant percentage of patients who die of cardiac sarcoidosis have no pre-mortem symptoms [2]. Therefore, it is important to screen for cardiac involvement in patients with sarcoidosis in hopes to identify and treat patients at highest risk of adverse cardiac events. This section will explore which patients should be evaluated by advanced multimodality imaging for cardiac sarcoidosis.
A number of studies have attempted to identify the percentage of patients with sarcoidosis who have cardiac involvement. These studies are confounded by the lack of a gold standard for the diagnostic criteria for cardiac sarcoidosis. For example, the 1993 and 2006 versions of the Japanese Ministry of Health and Welfare Diagnostic Criteria for cardiac sarcoidosis are insensitive for detecting cardiac involvement and do not risk stratify patients in the same manner as advanced imaging with either cardiac MRI (CMR) [3] or 18F-flurodeoxyglucose PET [4, 5]. In addition, unless a positive endomyocardial biopsy is obtained, these criteria require the presence of extra cardiac disease in order to establish the diagnosis of cardiac sarcoidosis. Therefore, it is extremely difficult to establish the diagnosis of isolated cardiac sarcoidosis using these criteria.
While there is no prospective data identifying the optimal screening strategy among patients with sarcoidosis, there is data to suggest that non-invasive screening strategies including evaluations of symptoms (palpitations, syncope, or pre-syncope), ECG (AV block, right bundle branch block, left anterior fascicular block, left posterior fascicular block, abnormal T waves), echocardiography (LVEF < 45 %, regional wall motion abnormalities, left ventricular hypertrophy, focal aneurysms), or Holter monitor (PVCs > 10/h, non-sustained ventricular tachycardia, Supraventricular tachycardia) can be used as a trigger to pursue advanced imaging with CMR and/or FDG PET with myocardial perfusion imaging.
The most common indication for screening patients with extra-cardiac sarcoidosis is the development of new cardiac signs or symptoms. Three studies have evaluated the utility of abnormal results on screening strategies to predict CMR and FDG PET abnormalities in patients with extra-cardiac sarcoidosis. Mehta et al. [6] evaluated the ability of symptoms, ECG, echocardiogram, and Holter monitoring to predict the presence of abnormal imaging findings by either CMR or FDG PET. In their cohort of 62 patients with biopsy-proven extra-cardiac sarcoidosis, cardiac symptoms (46 vs. 5 %), abnormal Holter monitoring (50 vs. 3 %), and abnormal echocardiogram findings (25 vs. 5 %) were more common in patients who had abnormal CMR or FDG PET studies. Nearly half (47 %) of patients had some abnormality on baseline screening testing, and of those with abnormal screening testing 88 % had abnormal FDG PET and 36 % had abnormal CMR studies. Collectively, the sensitivity of any of the above screening variables to detect cardiac sarcoidosis was 100 % and the specificity was 87 %. Freeman et al. [7] used a more detailed screening regimen incorporating ECG (resting and signal averaged), echocardiogram, electrophysiology study (if performed), and nuclear stress testing to retrospectively evaluate 70 patients with systemic sarcoidosis. They found increasing numbers of abnormal screening studies were associated with a greater likelihood of abnormal CMR or FDG PET results, and that the absence of abnormal screening results lead to a lower rate (2/18, 11 %) of abnormal advanced imaging. In both the studies by Mehta and Freeman, a greater number of abnormal screening tests lead to an increased likelihood of abnormal advanced imaging. Consistent with these findings, recent data from a small study has showed that among patient without cardiac symptoms and normal LV systolic function on echocardiography, the rate of abnormal findings on CMR imaging was low (13 %) [8]. Collectively, the above data can be used to support the use of cardiac symptoms, abnormal ECGs, or abnormal echocardiographic findings for pursuing advanced imaging testing in patients with extra-cardiac sarcoidosis. The Heart Rhythm Society Expert Consensus Statement on Arrhythmias Associated with Cardiac sarcoidosis has also endorsed this strategy [9].
There are certain other clinical scenarios that should prompt an evaluation for cardiac sarcoidosis even when there is no prior history of sarcoidosis. These include new onset unexplained advanced heart block in adults under 60 years of age and/or unexplained ventricular tachycardia. Among patients with new unexplained heart block, cardiac sarcoidosis may be present in 25–34 % [10, 11]. Furthermore, numerous reports have described unexplained sustained monomorphic ventricular tachycardia (VT) as the initial presenting symptom of cardiac sarcoidosis. In these scenarios, if the patient does not have known extra-cardiac sarcoidosis then the use of chest CT or whole-body FDG PET/CT imaging may identify previously clinically silent extra-cardiac sarcoidosis (i.e. hilar/mediastinal lymphadenopathy) that is more amenable to biopsy than the heart. While the identification of extra-cardiac sarcoidosis significantly increases the likelihood of cardiac sarcoidosis, it is important to recognize that isolated cardiac sarcoidosis – whereby the disease is confined only to the heart – is likely significantly under diagnosed and may carry a worse prognosis. Therefore, the absence of extra-cardiac disease cannot be used to exclude the possibility of cardiac sarcoidosis.
In summary, our recommendations for screening for cardiac sarcoidosis include:
1.
All patients with sarcoidosis should undergo a regular clinical evaluation which includes prompting for any new cardiac signs or symptoms. In addition, all patients should be screened with ECGs as part of their clinical care. Among selected patients, Holter monitoring can be performed if symptoms warrant. In addition, among selected patients, echocardiography can also be performed, realizing it is insensitive for detecting cardiac involvement or for predicting abnormal CMR or FDG PET findings (see next section).
2.
Any abnormal screening test, new cardiac sign or symptom, or high clinical suspicion, particularly unexplained heart block or VT, should prompt further evaluation with CMR and/or FDG PET with myocardial perfusion imaging.
However, it is important to acknowledge that the role of wider screening of all patients with sarcoidosis with CMR (which, as discussed below, is likely a better screening test than PET given its higher sensitivity and lower cost) remains unknown. On one hand, such an approach will identify more patients who have cardiac involvement. On the other hand, it is unknown if identifying and treating clinically silent disease will lead to any improvement in patient outcomes, and whether such approaches will be cost effective, particularly when also considering the cost of downstream testing that may occur.
Role of Multimodality Imaging in the Diagnosis of Cardiac Sarcoidosis
Imaging plays a critical role in the diagnosis of cardiac sarcoidosis and is particularly important due to the insensitivity of cardiac biopsy and the imprecision of clinical criteria in diagnosing cardiac sarcoidosis. A number of imaging techniques have been used to evaluate for cardiac sarcoidosis, including echocardiography, nuclear perfusion imaging, imaging of inflammation (FDG PET and gallium-67), and cardiac MRI (CMR). While most of these techniques have been reviewed in other chapters in this book, this section will provide a multi-modality overview of how these tests are used to diagnose cardiac sarcoidosis. In addition, we will present an algorithm for the use of advanced multimodality imaging for the evaluation of cardiac sarcoidosis, highlighting the complementary aspects of CMR and FDG PET with myocardial perfusion imaging (MPI).
Echocardiography
Echocardiography has been used to evaluate patients for sarcoidosis since the mid-1980s. Classic echocardiographic patterns for sarcoidosis involving the heart include the presence of basal septal thinning, depressed left ventricular ejection fraction, regional wall motion abnormalities (RWMAs), focal hypertrophy, or left ventricular aneurysms. These findings are neither sensitive nor specific for cardiac sarcoidosis and do not correlate well with CMR or FDG PET with MPI. For example, Mehta et al. found echocardiographic abnormalities in only 25 % of the patients who exhibited CMR or FDG PET evidence of cardiac sarcoidosis [6] and Freeman et al. determined a negative predictive value of only 32 % for echocardiography [7]. This corresponds to unpublished data from our own group suggesting that in patients with suspected cardiac sarcoidosis, echocardiograms are normal in as many as a quarter of patients who have FDG PET studies with abnormal perfusion and half of patients who have abnormal FDG uptake (Miller et al. unpublished data). Thus in our opinion, the low sensitivity and negative predictive value of echocardiography when compared to other techniques make it less suitable for use as a screening test for cardiac sarcoidosis, particularly among patients who have any of the aforementioned abnormalities that warrant screening.
Nuclear Imaging Techniques
Historically, nuclear imaging techniques such as thallium-201 or gallium-67 scintigraphy have been used to evaluate for abnormal cardiac perfusion/scar or inflammation, respectively. However, these have been supplanted by newer techniques. Specifically, PET perfusion tracers (rubidium-82 and N-13 ammonia) have increased sensitivity and specificity compared to thallium-201 or technetium-99m for detection of perfusion defects. Among patients with cardiac sarcoidosis, such defect may be due to scar and/or marked inflammation resulting in compression of the microvasculature. For the detection of cardiac inflammation, 18F-flurodeoxyglucose has superior diagnostic performance compared to gallium-67. Therefore, FDG PET performed with MPI has become the standard of care for nuclear imaging evaluation of cardiac sarcoidosis since it can evaluate both pathological processes of cardiac sarcoidosis (scar and inflammation).
Appropriate pre-scan patient preparation is essential in order to suppress the uptake of FDG from normal myocardium. Further details regarding this are available in Chap. 6. Based on the experience of our centers we suggest one of the following protocols:
A high fat/low carbohydrate diet for two meals prior to the scan followed by a fast of at least 4 h
A high fat/low carbohydrate diet followed by a fast of at least 12 h
Prior to imaging, it should be confirmed that the patients were able correctly follow the diet/preparation instruction.
The acquisition protocol for FDG PET with MPI for cardiac sarcoidosis is discussed in detail in Chap. 6 and is shown in Fig. 7.2. The study begins with a resting myocardial perfusion imaging study. When available, PET resting MPI (Rb-82 or N-13 NH3) is preferable, but SPECT MPI (Tc-99m) can also be performed, and should be combined with attenuation correction when available. Subsequently, the patient is injected with 10–12 mCi of F18- FDG. Following an uptake period of 90 min 3-D FDG PET acquisition of the heart and whole body is performed.
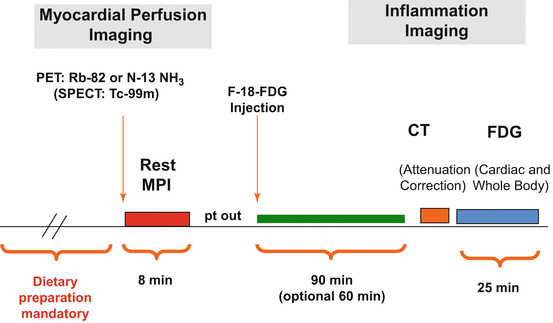

Fig. 7.2
Example of a FDG PET plus MPI protocol for cardiac sarcoidosis. Further details are provided in the text as well as in Chap. 7
The interpretation of FDG PET with MPI studies includes an evaluation of both perfusion and FDG uptake (Fig. 7.3). Perfusion defects are described using a traditional 0–4 grading scale for severity and 17-segment model for location. Abnormal FDG uptake is described as a focal or focal-on-diffuse area of the myocardial uptake with intensity greater than the left ventricular blood pool (see Fig. 7.4 for examples). In addition, it is important to describe whether there are focal areas of increased FDG uptake involving the right ventricle. Whole body FDG imaging using a large field of view from the base of the skull to the mid-thigh level should be performed in order to evaluate for extra-cardiac sarcoidosis.
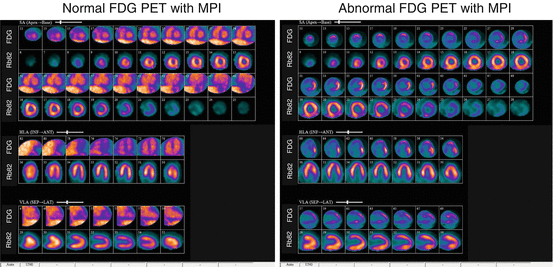
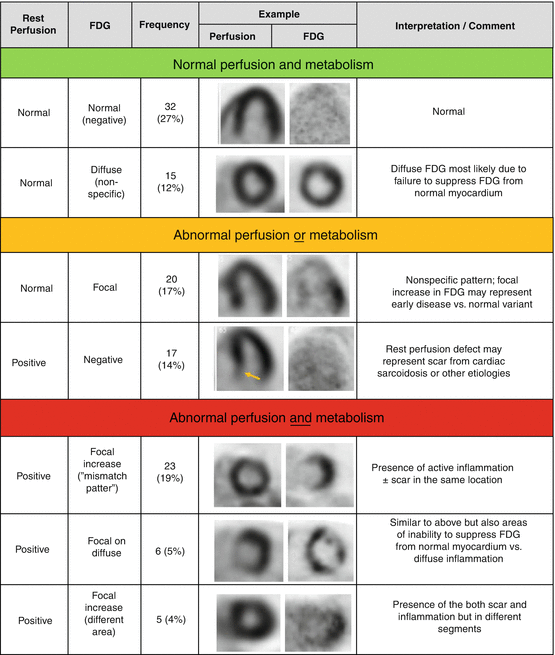

Fig. 7.3
Example of FDG PET with MPI images for the evaluation of cardiac sarcoidosis. (Left) Normal pattern for fasting FDG images in the heart, showing no myocardial uptake, but faint FDG signal present in the LV blood pool. Rb82 perfusion is normal. (Right) Abnormal FDG PET with MPI showing a focal area of FDG uptake with greater intensity than LV blood pool in the basal lateral/infero-lateral walls. In addition, there is a focal basal infero-lateral perfusion defect on the Rb82 images. This highlights the complementary information gained by use of FDG (inflammation) and Rb82 (scar) imaging for the evaluation of cardiac sarcoidosis

Fig. 7.4
Classification of cardiac PET/CT perfusion and metabolism imaging. (With permission from Elsevier Publication. Source: Blankstein et al. [5])
While cardiac sarcoidosis may cause isolated FDG uptake without any perfusion defects, or in more advance stages of disease, resting perfusion defects without any FDG uptake, the most characteristic finding is a perfusion/metabolism “mismatch” whereby areas of increased FDG also exhibit a resting perfusion defect. It is noteworthy that such a mismatch pattern can also occur in patients with hibernating myocardium, and thus when the diagnosis of cardiac sarcoidosis is suspected based on such PET findings, it may be necessary to rule out the presence of obstructive coronary artery disease (CAD). Nevertheless, at times, there are challenging cases where CAD and cardiac sarcoidosis can both be present.
Recently, newer methods of quantitative interpretation of FDG uptake including the volume of inflammation [12] and integrated volume-intensity [4] have been described. Such measures are most useful when comparing scans in order to follow response to therapy, but may also enhance the standardization of interpretation among centers.
While there are wide ranges of estimates for the sensitivity of FDG PET with MPI for detecting cardiac sarcoidosis, these estimates are rather misleading given the lack of a reference gold standard criteria and the fact that imaging is more sensitive for detecting disease than the Japanese criteria. With these important limitations in mind, a recent meta-analysis involving a total of 164 patients from 7 studies described a sensitivity of 89 % and specificity of 78 % when compared against the 2006 JMHW criteria [13]. However, the reduced specificity of PET in these studies may be due to the fact that it is more sensitive than the reference criteria against which it was compared.
Cardiac MRI
Cardiac MRI is also an important and useful advanced cardiac imaging technique used for the evaluation for cardiac sarcoidosis. CMR is a highly accurate technique to evaluate cardiac structure and function, and it can be used to define morphological features of cardiac sarcoidosis analogous to echocardiography (e.g. regional wall motion abnormalities, wall thinning, hypertrophy, LV systolic dysfunction, and focal aneurysms). However, the major role for CMR in the diagnosis of cardiac sarcoidosis lies in the evaluation of late gadolinium enhancement (LGE) whereby T1 weighted inversion recovery images are acquired ~10 min following the administration of intravenous gadolinium. Gadolinium is an extracellular contrast agent that has a rapid washout from normal areas of myocardium. However, in patients with cardiac sarcoidosis, the presence of scar or intense inflammation can expand the extracellular space and result in slower washout of gadolinium and thus areas of increased T1 signal enhancement. Such areas of LGE – which commonly are sub-epciardial or midwall in location – may be seen even when there are no regional wall motion abnormalities and normal wall thickness. As a result, in patients with suspected cardiac sarcoidosis, the presence of LGE is more sensitive than echocardiography and perfusion imaging for the detection of scar formation.
While the typical pattern of LGE in cardiac sarcoidosis is a non-transmural pattern involving either the sub-epicardial and/or mid-myocardial walls (Fig. 7.4), patterns of subendocardial or transmural LGE (which may mimic common CAD patterns) have been described as well.
Similar to FDG PET, estimates of the accuracy of CMR to diagnose cardiac sarcoidosis are hampered by a lack of gold standard. The original report of the diagnostic accuracy of CMR for cardiac sarcoidosis showed a sensitivity of 100 % and specificity of 78 % versus the JMHW criteria [14] with the low specificity a consequence of comparison versus the insensitive JMHW criteria. This superiority of CMR for diagnosing cardiac involvement in sarcoidosis was borne out by Patel et al. who showed a greater than 2x increase in cardiac sarcoidosis diagnosis versus JMHW criteria, leading to a significant improvement in prognostication of adverse events by CMR when compared to JMHW criteria [14].
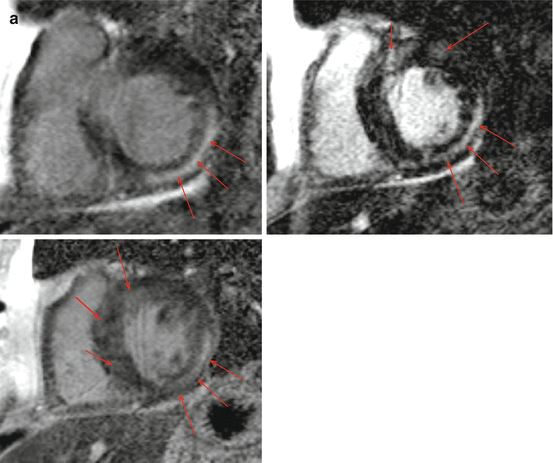
There is only a single small study that has directly compared CMR and PET, the results of which support the complementary value of these two techniques. In this study, Ohira et al. [15] evaluated 21 consecutive patients with suspected cardiac sarcoidosis who underwent both exams and found that 5 were negative by both modalities and 8 were positive by both. Interestingly, 8 patients had discordant findings: 7 had only PET abnormalities while 1 had only CMR abnormalities. Among the 8 (38 %) patients who were categorized as having CS by JMHW criteria, 3 had abnormal findings by only one modality while 5 had abnormalities on both. This study was underpowered to detect significant differences in diagnostic accuracy between these techniques, but has been incorrectly quoted by some to suggest that PET may have a higher sensitivity based on a non-significant, numerically higher sensitivity for PET [7 out of 8] than CMR [6 out of 8] (Fig. 7.5).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>






Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


