Despite important progress in recent decades, mortality remains high for patients with chronic heart failure. Risk stratification may be refined by the use of biomarkers for different pathophysiological processes that established mortality risk factors do not directly reflect. Biomarkers that are currently available can provide information about at least 7 pathobiological processes operative in HF, help to identify the specific processes involved in individual patients, and aid in constructing management plans. However, the additional prognostic information gained by any biomarker over a clinical risk model plus other biomarkers needs to be determined with adequate statistical tools. A major problem in selecting a biomarker profile is the proportional increase in economic burden; thus, the addition of any biomarker to a profile should be justified by adequate discrimination, calibration, reclassification, and likelihood analyses. Three studies that implemented such rigorous analyses have assessed a multimarker panel in chronic heart failure that incorporated the biomarker ST2: the Penn HF Study, the Barcelona Study, and the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) biomarker substudy. In all 3 studies, a multimarker panel appeared to provide significant information over conventional risk stratification. The latter 2 reports proposed that ST2 might be superior to natriuretic peptides. The Barcelona Bio-HF calculator ( www.bcnbiohfcalculator.cat ) is a novel risk calculator that considers clinical variables, treatment, and biomarkers (i.e., N terminal pro-brain natriuretic peptide [NT-proBNP], ST2, and high sensitivity troponin T [hsTnT]). The optimal panel of markers, the change in these markers over time, and how these changes might help guide therapeutic interventions remain to be defined.
Chronic heart failure (HF) is a growing public epidemic, with increasing incidence and prevalence. Despite important progress in recent decades, mortality remains high for patients with HF. Moreover, established risk factors, such as New York Heart Association (NYHA) functional class, medication use, routine laboratory values, and left ventricular ejection fraction (LVEF), do not fully explain the mortality risk of patients with HF and do not estimate the prognosis of individuals. Risk stratification may be refined by the use of biomarkers for different pathophysiological processes that established mortality risk factors do not necessarily directly reflect.
Although the definition of a biomarker includes virtually any measurement that can be made on a biologic system, this review is restricted to substances measured in the blood other than genetic markers, electrolytes, and commonly used markers of hepatic or renal function. These biomarkers aid in the diagnosis of HF, provide an estimate of prognosis, and help in the identification of apparently healthy people who are at excessive risk for HF. When assessing the clinical value of any individual biomarker, it is important to determine whether it provides independent incremental information when added to previously available information; this can be estimated by determining whether it increases discrimination ( c -statistic or area under the receiver operating characteristic curve [AUC]), as well as by calculating the net reclassification improvement index and the integrated discrimination improvement index.
Among the multiple biomarkers for HF, natriuretic peptides (NPs), ST2, and troponins deserve particular attention because of their strong prognostic potential in chronic HF and because they have been incorporated into the majority of multimarker strategies. NPs are of enormous clinical value in diagnosing HF in patients with dyspnea of unknown origin, in patients with heart disease without clinical manifestations of HF but who are at risk for it, and in apparently healthy patients who are at higher risk for HF. However, like any laboratory measurement, NP levels must be interpreted within the context of a patient’s characteristics, such as age and body mass, and other tests, including cardiac imaging.
ST2 is a protein that exists in soluble and membrane-bound forms; the latter form is the receptor for interleukin-33. When the myocardium is stretched, the ST2 gene is upregulated and the concentration of circulating soluble ST2 increases rapidly. The level of circulating ST2 has been reported to be a predictor for HF and death in patients with ST-segment elevation myocardial infarction, acute decompensated HF, and chronic HF. This biomarker provides prognostic information that is independent of and in addition to that offered by NPs, although the release of both seems to occur in response to the same stimulus: cardiac stretch.
As measured by standard assays, abnormal elevations of circulating troponins have been reported in about 25% of patients with HF; they denote a poor prognosis, generally defined as death or early readmission for HF. High-sensitivity assays (hsTn) detected abnormal elevations of circulating troponins in virtually all patients with acute decompensated HF and in a majority of a population with chronic HF.
In 2008, Braunwald classified circulating biomarkers into categories based on their pathophysiological effects in HF and hypothesized that multiple biomarkers in combination would provide a valuable means for risk stratification. The biomarkers that are currently available reflect at least 7 pathobiological processes operative in HF, help to identify the specific processes involved in individual patients, and aid in the design of management plans.
There has recently been interest in multimarker strategies to examine panels of biomarkers that assess different pathophysiological pathways. Consequently, multimarker approaches to predict the risk for mortality in patients with chronic HF have been described. To date, there are 3 published reports on multimarker strategies that incorporate ST2 in their panel.
In a recent study of ambulatory patients with chronic HF, the Penn HF Study (PHFS), Ky et al evaluated the predictive utility of a panel of biomarkers reflective of diverse biologic pathways in HF. The panel included high-sensitivity C-reactive protein (inflammation), uric acid and myeloperoxidase (oxidative stress), B-type natriuretic peptide (BNP; neurohormonal activation), soluble fms-like tyrosine kinase receptor-1 (sFlt-1; vascular remodeling), troponin I (myocyte injury), ST2 (myocyte stress and fibrosis), and creatinine (renal function). This was a multicenter cohort study of 1,513 ambulatory patients with chronic HF. The authors’ hypothesis was that a biomarker score that summarized the activity of multiple pathways implicated in HF would improve the ability to classify risk for adverse outcomes (cardiac transplantation, ventricular assist device placement, and death) compared with a validated clinical risk prediction algorithm, the Seattle HF Model (SHFM).
The PHFS found that a multimarker score composed of 7 biomarkers (each reflecting a different pathophysiological pathway) was a strong predictor for risk and significantly improved the prediction of outcomes compared with the most commonly used clinical risk score in HF, the SHFM. Patients in the highest multimarker tertile had a nearly 14-fold unadjusted risk of death, transplant, or ventricular assist device placement compared with those in the lowest tertile. This risk remained nearly sevenfold after adjustment for the SHFM. The multimarker score showed a substantial ability to discriminate individual patient risk at 1 year (AUC 0.798) that was again superior to the SHFM. Addition of the multimarker score to the SHFM appropriately reclassified a large proportion (24.1%) of patients as higher risk. Although these findings support the concept of a multimarker tool for prognosis, identifying the “optimal” panel of biomarkers for assessing HF remains a formidable task. Substantial correlations among many of the biomarkers measured were found, strongly suggesting that there is no single “optimal panel.”
Next, Lupón et al, in a real-life ambulatory cohort of 876 consecutive patients (the Barcelona Study; median age 70.3 years, LVEF 34%), investigated different biomarker combinations of N terminal pro-brain natriuretic peptide (NT-proBNP), ST2, and high sensitivity troponin T (hsTNT) to determine the relative role of each in chronic HF risk stratification. All 3 biomarkers were incorporated into a model with 11 well-established risk factors: age, gender, ischemic etiology, LVEF, NYHA functional class, diabetes mellitus, estimated glomerular filtration rate, sodium level, hemoglobin level, β-blocker treatment, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker treatment. The AUC for this model was 0.76, which compares favorably with other validated models for HF, such as the SHFM. The combined addition of hsTnT and ST2 to the model yielded good measures of performance (AUC 0.789) and a net reclassification index of approximately 14%. Importantly, reclassification did not improve after the addition of NT-proBNP to the full model.
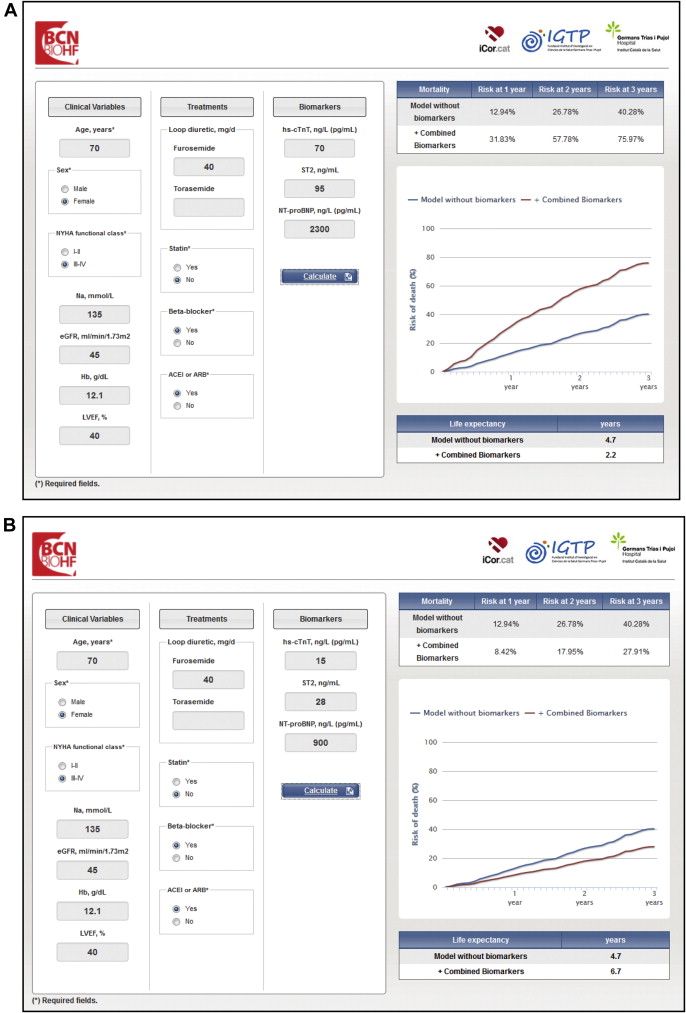
The utility of combining NPs with either ST2 or hsTnT has been reported previously, but not the combination of the 3 biomarkers in the setting of chronic HF. The different analyses of this study yielded 3 relevant findings. First, NT-proBNP added to hsTnT + ST2 did not improve prognostic accuracy or reclassification indexes. Second, NT-proBNP increased prognostic discrimination only in patients with either hsTnT or ST2 levels below the cut-off point. Third, the combination of hsTnT + ST2 identified more decedents during follow-up than did the combination of the 3 biomarkers. Together, these main findings suggest that the pathways identified by ST2 and hsTnT profoundly affect mortality in the context of chronic HF, whereas the information provided in their presence by NPs may be redundant. The investigators used these data to develop a novel HF risk calculator, the Barcelona Bio-HF calculator (BCN Bio-HF calculator; www.bcnbiohfcalculator.cat ). The BCN Bio-HF calculator is a Web-based calculator that allows for quick and easy interactive calculations of prognosis at 1, 2, and 3 years, as well as life expectancy ( Figure 1 ). The BCN Bio-HF calculator includes 3 commercially available complementary biomarkers that provide information about myocyte necrosis (hsTnT); fibrosis, remodeling, and inflammation (ST2); and chamber strain (NT-proBNP). The calculator was developed with 8 models that include none, 1, 2, or 3 of the biomarkers, allowing it to be used with any combination of biomarkers. This characteristic is unique to this new tool. In combination with the use of state-of-the-art statistics for biomarker values including c -statistic, as well as calibration and reclassification, the inclusion of these 3 biomarkers makes the calculator more robust. Internal and external validation of the BCN Bio-HF calculator were reported recently.