White matter hyperintensity
Cerebral microbleeds
Recent small subcortical infarct
Lacunes
Dilated perivascular space
Usual diameter
Variable
≤10 mm
≤20 mm
3–15 mm
≤2 mm
Comment
Located in the white matter
Detected on GRE, round or ovoid, blooming
Best identified on DWI
Usually hyperintense rim
Mostly linear without hyperintense rim
DWI
↔
↔
↑
↔/(↓)
↔
FLAIR
↑
↔
↑
↓
↓
T2
↑
↔
↑
↑
↑
T1
↔/(↓)
↔
↓
↓
↓
T2-weighted GRE
↑
↓↓
↔
↔/↓ if hemorrhage
↔
17.1.1 White Matter Hyperintensity
17.1.1.1 Definition
WMHs of presumed vascular origin are bilateral, mostly symmetrical hyperintense lesions on T2-weighted sequences of variable size in the white matter. On T1 sequences, WMH can appear as isointense or hypointense (Fig. 17.1, panel a). Lesions in the subcortical gray matter or brainstem are not included in this category. When using CT, white matter hypoattenuation or white matter hypodensities can be detected. CT and MRI rating scales for WMH have been suggested [3].
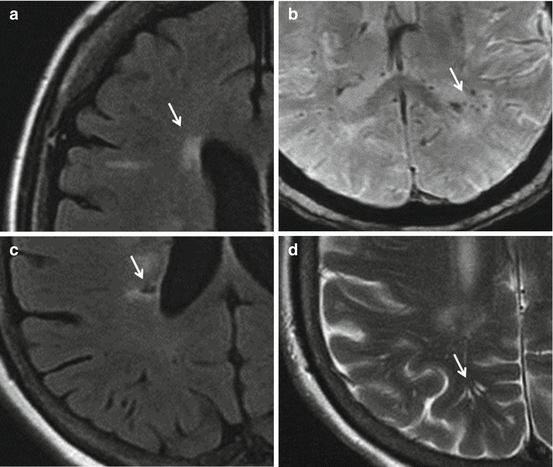

Fig. 17.1
Panel (a) Periventricular white matter hyperintensity – fluid-attenuated inversion recovery (FLAIR) MRI. Panel (b) Microbleeds – susceptibility-weighted imaging (SWI) MRI. Panel (c) Lacunes – fluid-attenuated inversion recovery (FLAIR) MRI. Panel (d) Perivascular space – T2-weighted turbo spin-echo MRI. Lesions are shown by arrows (MRI scans are courtesy of Dr. Zuzana Ryznarova, Dept of Radiology, Thomayer Hospital, Prague, Czech Republic)
17.1.1.2 Epidemiology
17.1.1.3 Association with Hypertension
Hypertension is currently considered the most important modifiable risk factor for WMH volume and progression [6]. Midlife BP and cumulative systolic blood pressure (BP) and cumulative systolic BP measured over a period of several years seem to be better predictors of WMH than single late-life measurements. Hypertension or a history of hypertension has been associated with a greater WMH volume in the frontal lobe, indicating greater susceptibility of the frontal lobe to the hypertension-related white matter degradation. An association between systolic BP and injury to the white matter microstructure was described already in young adults in their 30s, suggesting that these lesions develop insidiously during life [7].
There is mounting evidence that BP control may reduce the course of WMH progression. In the 3C-Dijon MRI study, baseline BP and BP control over a 4-year follow-up were strong predictors of WMH volume progression [8]. However, the association with BP was only present for total and periventricular WMH. In the PROGRESS MRI substudy, a combination of perindopril plus indapamide was able to reduce WMH progression over a period of 3 years as compared to placebo [9]. The rate of WMH progression is lower in controlled treated hypertensives than in uncontrolled treated or untreated hypertensives [10].
17.1.1.4 Prognostic Value
According to a meta-analysis of 22 longitudinal studies, WMH is an independent indicator of future risk, which triples the risk of stroke and doubles the risk of dementia and death [5]. Extensive WMHs predispose to both ischemic (especially SSI) and hemorrhagic strokes. Moreover, WMHs are associated with covert neurological and cognitive symptoms and physical difficulties such as gait disturbance [11].
17.1.2 Cerebral Microbleeds (MBs)
17.1.2.1 Definition
Cerebral MBs are small (generally 2–5 mm but, occasionally, up to 10 mm in diameter) areas of signal void with associated blooming seen on T2* gradient-recalled echo (GRE) and susceptibility-weighted (SWI) MRI sequences and correspond to areas of hemosiderin breakdown from prior microscopic hemorrhage (Fig. 17.1, panel b). These are commonly caused by hypertensive vasculopathy or cerebral amyloid angiopathy. MRI is able to detect bleeding from vessels as small as 200 μm in diameter. The patterns of MB distribution differ by etiology. MBs in deep subcortical or infratentorial areas are usually associated with the presence of hypertension or vascular risk factors, with lipohyalinosis being the predominant finding. MBs in lobar and cortico-subcortical areas are associated with apolipoprotein E carrier status (ε4), β-amyloid burden, and evidence of cerebral amyloid angiopathy. Low total cholesterol and statin use have been found to be associated with increased risk of lobar MBs. Lacunes and WMH are associated with the deep or infratentorial MBs, but not with those in a lobar region.
17.1.2.2 Epidemiology
MB prevalence gradually increases with age, from 6.5 % in persons aged 45–50 years to 36 % in individuals ≥80 years of age [12]. In patients after stroke, MBs are present more often in patients with hemorrhagic stroke as compared with ischemic stroke. Of ischemic stroke subtypes, MBs occur more often in SSI than in other stroke subtypes. MBs are also more prevalent in subjects with mild cognitive impairment, Alzheimer’s disease, and vascular dementia, as compared with subjects with cognitive normal controls. Lovelock et al. demonstrated an excess of MB in warfarin users compared with no antithrombotic users and in warfarin-related intracranial hemorrhage versus spontaneous intracranial hemorrhage, suggesting that MB may increase the risk of warfarin-associated intracranial hemorrhage [13].
17.1.2.3 Association with Hypertension
Hypertension is independently associated with cerebral MBs both in otherwise healthy adults and in adults with cerebrovascular diseases [14]. Among patients with hypertension, 24-h systolic, diastolic, and pulse pressure are independent predictors of MB progression. In subjects after lacunar stroke, higher BP is associated with the development of a new MB; however, a decrease in BP during follow-up does not halt MB progression [15]. Thus, it is not clear whether optimal BP control can slow down progression of MB.
17.1.2.4 Prognostic Value
The presence of MBs is associated with increased risk of incident intracerebral hemorrhage, particularly in patients on anticoagulation therapy. Among patients after ischemic stroke, the presence of MBs significantly increases the risk of recurrent hemorrhagic and ischemic stroke [16]. In the PROSPER study over 7-year follow-up, MBs were associated with a sixfold increased risk of stroke-related death. Those with non-lobar MBs had double the risk of cardiovascular disease-related death independently of other vascular risk factors, whereas those with lobar MBs had a sevenfold increase in the risk of stroke-related death, but not cardiovascular death [17]. In the Rotterdam study, the presence of MBs, and particularly deep or infratentorial MBs, was independently associated with an increased risk of all-cause and cardiovascular mortality [18].
17.1.3 Recent Small Subcortical Infarct
17.1.3.1 Definition
Recent small subcortical infarct (SSI) is defined as a neuroimaging evidence of recent infarction in the territory of one perforating arteriole, with imaging features or clinical symptoms consistent with a lesion occurring in the previous few weeks. The size of the lesion should be ≤20 mm in its maximal diameter in the axial plane. Acute lesions can shrink to leave lacunes, WMH, or completely disappear. A recent study has shown 97 % cavitation of SSI at 90 days [19]. Historically, SSI is commonly called as lacunar stroke or lacunar syndrome. This nomenclature is derived from the French expression for a small fluid-filled cavity (lacuna) that was thought to mark the healed stage of an SSI. Thus, the pre-cavitary stage became the lacunar infarct. As noted above, not all SSIs transform into lacunes, making the term lacunar stroke inaccurate. That is why STRIVE recommend to use the term SSI instead.
The primary location of SSI in the primary motor or sensory pathways explains why these lesions are clinically symptomatic despite their size, while other VBI lesions accumulate silently. An incidentally asymptomatic SSI can be found by chance and is referred to as a silent cerebral infarction.
17.1.3.2 Epidemiology
Clinically evident recent SSI is responsible for about 25 % of all ischemic strokes. Only about 50 % of recent SSIs are visible on CT, while at least 70 % can be found on DWI MRI. The incidence of lacunar strokes increases with age.
17.1.3.3 Association with Hypertension
Hypertension is the most prevalent risk factor for stroke, particularly for SSI. The prevalence of hypertension in patients with SSIs is higher as compared with other ischemic stroke subtypes [20, 21]. However, the mechanisms by which hypertension leads to SSI and other forms of cerebral SVD are largely unknown. Increased pressure pulsatility, impaired autoregulation of cerebral blood flow, and pulse wave encephalopathy (all of which emphasize the central hemodynamic role of aortic stiffness) have been suggested as possible mechanisms. Ischemic SSIs are caused mostly by cerebral arteriolar occlusive disease (e.g., arteriolosclerosis, lipohyalinosis, fibrinoid necrosis, and arteritis), while, in approximately 10 % of cases, occlusion of the perforating arteriole is caused by an embolus from a proximal source. A minority of SSIs are caused by hemorrhage due to amyloid angiopathy, fibrinoid necrosis, and arteritis.
Many clinical trials and meta-analyses have confirmed that BP lowering reduces the risk of stroke in primary and secondary prevention [22]. In the PROGRESS trial with patients after a previous cerebrovascular event, antihypertensive treatment reduced the risk of lacunar stroke by 23 %. In the recent SPS3 trial [23], lowering of systolic BP to a target of <130 mmHg in patients with a recent SSI resulted in nonsignificant reductions in all stroke, disabling or fatal stroke, and major vascular events and a significant reduction in intracerebral stroke, as compared with the treatment target of systolic BP 130–140 mmHg. This suggests that patients after SSI may benefit from lower BP targets.
17.1.3.4 Prognostic Value
Due to its size, SSI is commonly perceived to be associated with lower risk as compared with other ischemic stroke subtypes. While the early mortality and stroke recurrence risk are higher among patients with other stroke subtypes, the long-term risks and the risks of cardiac outcomes are similar [24]. This may be explained by the associated VBI. Furthermore, SSI is associated with increased risk of cognitive impairment and dementia. In a recent meta-analysis, approximately 30 % of patients were cognitively impaired at 4 years following an SSI, a similar proportion to non-SSI stroke.
17.1.4 Lacunes of Presumed Vascular Origin
17.1.4.1 Definition
A lacuna of presumed vascular origin is a round or ovoid, subcortical, fluid-filled cavity of between 3 and 15 mm in diameter. On fluid-attenuated inversion recovery (FLAIR) images, lacunes have a central cerebrospinal fluidlike hypointensity with a surrounding rim of hyperintensity (Fig. 17.1, panel c). However, the rim is not always present. Lacunes can be caused by a previous SSI (symptomatic), silent brain infarction (asymptomatic), or hemorrhage in the territory of one perforating arteriole. Old striato-capsular strokes, which are caused by atherosclerotic or embolic occlusion of several penetrating arteries and are >20 mm in the acute phase, can shrink to form a lacunar-like cavity. This may preclude discrimination of the etiology of old lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


