Although an increased pulmonary trunk (PT) diameter to ascending aorta (AA) diameter ratio (PT/AA ratio) is associated with pulmonary hypertension, the prognostic utility of this metric remains unexamined. We investigated whether an increase in the PT/AA ratio, as measured using coronary computed tomographic angiography, is associated with the risk of all-cause death. We identified 1,326 consecutive patients (mean age 61 ± 13 years; 60% men) without known coronary artery disease who underwent coronary computed tomographic angiography. Patients with a history of congenital or valvular heart disease or aortic enlargement (≥4 cm) were excluded. The PT and AA diameters were measured at the PT bifurcation level. The patients were categorized by PT/AA deciles, with the ≥90th percentile (PT/AA ratio 0.9) considered elevated. All-cause death associated with a PT/AA ratio <0.9 versus ≥0.9 was evaluated using multivariate Cox proportional hazard models. During 2.9 ± 1.0 years of follow-up, 58 patients died. Patients with a PT/AA ratio ≥0.9 experienced 2.5-fold greater annualized mortality compared to those with <0.9 (3.1% vs 1.3%, p = 0.004). Adjusting for age, gender, heart rate, dyslipidemia, smoking, and coronary artery disease extent, the patients with a PT/AA ratio ≥0.9 experienced a greater mortality risk compared to patients with PT/AA ratio <0.9 (hazard ratio 3.2, 95% confidence interval 1.6 to 6.6, p = 0.001). In the 1,059 patients with left ventricular ejection fraction measurements, a lower left ventricular ejection fraction was observed in the PT/AA ratio ≥0.9 group (p <0.05). In conclusion, incrementally and independent of the traditional coronary artery disease risk factors, an elevated PT/AA ratio was associated with increased mortality risk in patients without known coronary artery disease undergoing coronary computed tomographic angiography.
Coronary computed tomographic angiography (CCTA) is a rapidly increasing modality for the assessment of patients with suspected coronary artery disease. The pulmonary trunk (PT) and ascending aorta (AA) are routinely visualized using CCTA; however, their dimensions have not been universally reported. Previous studies have reported that an increased PT/AA diameter ratio on computed tomographic scans is associated with pulmonary artery hypertension (PAH). However, the prognostic potential of the PT/AA ratio remains inadequately unexplored. The aim of the present study was to determine whether the PT/AA ratio, as measured using CCTA, is associated with a risk of all-cause death in patients undergoing evaluation for suspected coronary artery disease.
Methods
We identified 1,326 consecutive patients (mean age 61 ± 13 years; 60% men) without known coronary artery disease, who underwent CCTA from September 2006 to October 2010 at the Cedars Sinai Medical Center (Los Angeles, California). Subjects with a history of myocardial infarction, previous coronary revascularization, congenital heart disease, valvular disease, aortic aneurysm, or the presence of ascending aortic enlargement (ascending aortic diameter ≥4.0) were excluded.
The PT/AA ratio using CCTA was defined as elevated when it was >90th percentile in the study population. On a gender-specific basis, this 90th percentile for the PT/AA ratio was similar for men and women (0.94 and 0.92, respectively). In accordance with this observation and previous work, 0.9 was identified as the optimal cutoff for an elevated PT/AA ratio for both men and women. In the study cohort, 1,144 patients possessed a PT/AA ratio <0.9 and constituted the normal group, and 182 had a PT/AA ratio of ≥0.9, constituting the abnormal group.
Weight, height (for calculation of body mass index and body surface area), blood pressure, and heart rate were collected. The traditional risk factors, including hypertension, dyslipidemia, diabetes, active smoking, and a family history of premature coronary artery disease, were documented, as previously described. All patients completed a questionnaire regarding chest pain characteristics (asymptomatic, atypical, nonanginal, and typical chest pain). The subjects reporting any shortness of breath without any chest pain were defined as having dyspnea. The institutional review board of the Cedars-Sinai Medical Center approved the present study.
A dual-source computed tomographic scanner (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany) was used for CCTA, as previously described. When needed, β blocking was used to achieve the target heart rate (≤70 beats/min), and sublingual nitroglycerin (ScielePharma, Alpharetta, Georgia) was administered for coronary artery dilation. The images were acquired after 85-ml contrast injection (Omnipaque or Visipaque, GE Healthcare, Princeton, New Jersey) using either prospective axial or helical scanning, with dose modulation and a tube voltage of 120 or 100 kVp. The coronary artery calcium score was assessed using noncontrast-enhanced computed tomography in 1,156 patients. In 1,059 subjects who underwent helical CCTA, the left ventricular (LV) end-diastolic volume, end-systolic volume, and ejection fraction were measured.
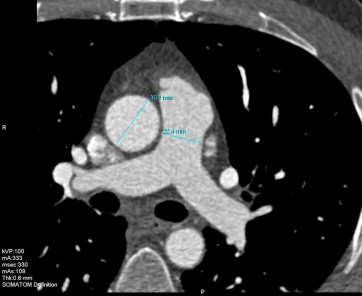
Two experienced readers evaluated the coronary computed tomographic angiographic scans for the presence, extent, and severity of coronary artery plaque using the interactive axial and oblique views and curved multiplanar reconstructions. In the case of discordant findings, the final decision was made by consensus. The AA dimension was measured for the endoluminal diameter in the transverse axial plane at the level of the PT bifurcation during a mid-diastolic phase (70% phase), similar to that for coronary artery assessment. The PT diameters were measured using the same method ( Figure 1 ).
Detected coronary plaques were assigned locations according to a modified 17-segment American Heart Association coronary tree model. Coronary plaque was identified as any hyperdense structure of any size adjacent to the lumen or any hypodense structure distinct from lumen and periarterial tissue >1 mm 2 in the largest cross-sectional area. The coronary artery stenosis severity was classified into 4 groups (0%, 1% to 49%, 50% to 69%, and ≥70% stenosis), in accordance with societal guidance documents. On a per-patient basis, the maximum stenosis severity was defined as the most severe stenosis in any coronary artery segment ≥1.5 mm in diameter. As previously described, we examined the overall coronary artery plaque distribution using a segment involvement score, which was defined as the number of coronary artery segments with any plaque.
Continuous variables are expressed as the mean ± SD. The unpaired Student t test or the Wilcoxon rank-sum test was used to conduct the normal and abnormal ratio group comparison. Categorical variables were compared using the Pearson chi-square test. All-cause death was determined from the Social Security Death Index. The annual event rates were calculated and compared using the log-rank test. A multivariate Cox proportional hazard model was used to assess the PT/AA ratio ≥0.9 for prediction of all-cause death after adjusting for age, gender, heart rate, dyslipidemia, smoking, and segment involvement score. For the 1,059 subjects with an LV ejection fraction measurement, a multivariate Cox proportional hazard model was used to investigate the PT/AA ratio ≥0.9 for predicting all-cause death after adjusting for age, gender, smoking, LV end-diastolic volume, and reduced LV ejection fraction (i.e.,<45%). All statistical calculations were performed using STATA, version 11 (StataCorp, College Station, Texas) for Windows. p Values <0.05 were considered statistically significant.
Results
The baseline characteristics of the patients in the cohort are listed in Table 1 . The patients with an abnormal PT/AA ratio were younger, more frequently smokers, more likely to present with dyspnea, and had a greater baseline heart rate than those with a normal PT/AA ratio. In contrast, the patients with a normal PT/AA ratio had a greater prevalence of dyslipidemia and were more likely than patients with an increased PT/AA ratio to be treated with aspirin or statins. Compared to the patients with a normal PT/AA ratio, those with an abnormal PT/AA ratio had a larger overall PT diameter (2.9 ± 0.4 vs 2.4 ± 0.3 cm, p <0.0001) and a smaller AA diameter (2.9 ± 0.4 vs 3.2 ± 0.4 cm, p <0.0001). Of the 1,059 subjects with an LV ejection fraction, compared to the patients with a normal PT/AA ratio, those with an abnormal PT/AA ratio had a lower LV ejection fraction (67.5 ± 9.8% vs 64.0 ± 12.7%, p = 0.008) and a greater prevalence of LV ejection fraction <45% (2.5% vs 7.7%, p = 0.001). The LV end-diastolic volume did not differ between the normal and abnormal groups (129.6 ± 36.2 vs 141.6 ± 53.6 ml, p = 0.08).
| Variable | Ratio Group | p Value | |
|---|---|---|---|
| Normal (n = 1,144) | Abnormal (n = 182) | ||
| Age (yrs) | 61 ± 13 | 56 ± 15 | <0.0001 |
| Men | 61 (%) | 58 (%) | 0.45 |
| Body mass index (kg/m 2 ) | 27 ± 5 | 28 ± 6 | 0.22 |
| Body surface area (m 2 ) | 1.94 ± 0.26 | 1.97 ± 0.28 | 0.28 |
| Heart rate (beats/min) | 65 ± 12 | 68 ± 12 | 0.002 |
| Systolic blood pressure (mm Hg) | 132 ± 20 | 129 ± 21 | 0.06 |
| Diastolic blood pressure (mm Hg) | 74 ± 25 | 72 ± 13 | 0.17 |
| Hypertension | 49 (%) | 47 (%) | 0.67 |
| Diabetes mellitus | 13 (%) | 14 (%) | 0.64 |
| Dyslipidemia | 62 (%) | 52 (%) | 0.02 |
| Smoking | 13 (%) | 19 (%) | 0.02 |
| Family history | 40 (%) | 41 (%) | 0.71 |
| Asymptomatic | 38 (%) | 37 (%) | 0.88 |
| Atypical angina | 24 (%) | 24 (%) | 0.93 |
| Nonangina | 9 (%) | 6 (%) | 0.09 |
| Typical angina | 18 (%) | 15 (%) | 0.27 |
| Dyspnea | 11 (%) | 18 (%) | 0.004 |
| Medication | |||
| Aspirin | 56 (%) | 47 (%) | 0.02 |
| Angiotensin-converting enzyme inhibitor and/or angiotensin II receptor blocker | 31 (%) | 34 (%) | 0.58 |
| β Blocker | 25 (%) | 27 (%) | 0.51 |
| Calcium channel blocker | 12 (%) | 9 (%) | 0.37 |
| Nitrate | 7 (%) | 5 (%) | 0.36 |
| Statin | 46 (%) | 33 (%) | 0.001 |
| Diuretic | 18 (%) | 20 (%) | 0.49 |
The coronary plaque characteristics among the normal and abnormal ratio groups are listed in Table 2 . Compared to the abnormal group, the normal ratio patients had a greater segment involvement score. The severity of coronary artery stenosis and extent did not differ significantly between the normal and abnormal PT/AA ratio groups ( Table 2 ). In 1,156 subjects with available coronary artery calcium scans, those with a normal PT/AA ratio had significantly greater coronary artery calcium scores than those with an abnormal PT/AA ratio (271 ± 573 vs 206 ± 445, p = 0.01).
| Variable | Ratio Group | p Value | |
|---|---|---|---|
| Normal (n = 1,144) | Abnormal (n = 182) | ||
| Segment involvement score | 3.0 ± 3.2 | 2.3 ± 3.1 | 0.004 |
| Maximum diameter stenosis | |||
| 0% | 33.0 (%) | 41.8 (%) | 0.02 |
| 1–49% | 48.0 (%) | 41.2 (%) | 0.09 |
| 50–69% | 7.8 (%) | 5.5 (%) | 0.28 |
| ≥70% | 11.2 (%) | 11.5 (%) | 0.89 |
| Coronary artery disease vessels (n) | |||
| 1 | 8.5 (%) | 8.8 (%) | 0.89 |
| 2 | 1.9 (%) | 1.7 (%) | 0.80 |
| 3 or left main | 0.8 (%) | 1.1 (%) | 0.67 |
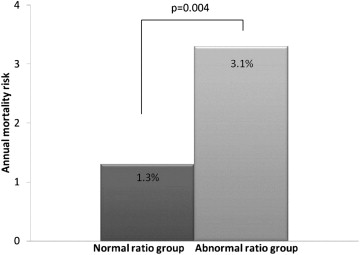
Of the 1,326 patients, 58 died (4.4% of total study population) during a follow-up period of 2.9 ± 1.0 years. Of these 58 deaths (4.4% of total), 43 were in the normal PT/AA ratio group compared to 15 in the PT/AA ratio ≥0.9 group. The overall all-cause death rate was greater in the abnormal PT/AA ratio group (8.2% vs 3.8%, p = 0.006), as was the annualized all-cause death rate ( Figure 2 ). In the multivariate Cox proportional hazards model adjusted for age, gender, dyslipidemia, smoking, heart rate, and segment involvement score, the patients in the abnormal PT/AA ratio group experienced a greater mortality risk than those in the normal PT/AA ratio group ( Table 3 and Figure 3 ). Of the 1,059 subjects with an LV ejection fraction measurement, 49 (4.6% of total) died: 40 in the normal PT/AA ratio group and 9 in the abnormal PT/AA ratio group (4.4% vs 6.3%, p = 0.31). In the multivariate Cox proportional hazards model adjusted for age, gender, smoking, LV end-diastolic volume, and LV ejection fraction <45%, an abnormal PT/AA ratio was associated with future mortality (hazard ratio 2.2, 95% confidence interval 1.1 to 4.7, p = 0.04).