Fig. 51.1
The Notch receptor is present in endocardial cells prior to EMT and will bind to its ligand and release its NCID domain. This domain forms a nuclear complex with MAML and RBPJK and will upregulate SNAIL, which leads to EMT formation. Simultaneously, myocardial BMP2 signals will reach the endocardium and will stabilize the nuclear SNAIL signal. At the correct level, VEGF produced by the myocardium will bind to its receptor on the endocardium and result in an increase of Ca2+ from the ER via IP3. This Ca2+, in concert with calmodulin, will activate calcineurin. Calcineurin will dephosphorylate NFATC1 and uncover its nuclear localization signal. It is believed that the step of NFATC1 dephosphorylation is also controlled by Creld1. Once in the nucleus, NFATC1 will bind to GATA4 and/or GATA5 among other proteins and will induce EMT
51.3.1 TGF-ß/BMP Family of Growth Factors
Aside from their vital role in heart development, the TGF-ß and BMP pathways play an essential role in embryonic development. In fact, the TGF-ß and the BMP subfamilies of secreted proteins are part of larger superfamily of growth factors known as the TGF-ß superfamily. This superfamily includes three major branches: the TGF-ß sensu stricto (TGF-ß in vertebrates), DVR (decapentaplegic-Vg-related, which includes BMP), and activin. These three different subfamilies share many characteristics such as the structure of the ligands, the receptors, the mechanism of action of the ligand-receptor complex, as well as the intracellular mediators of the ligands – the SMADs [8]. This similarity in the mechanism of action, especially in the SMAD mediators, will play an important role in the overlap between the BMP and the TGF-ß pathways as they relate to TA, as we shall discuss below.
Ligands of the TGF-ß superfamily require two receptors to function, TGF-ß receptors type I and II, both of which are receptor tyrosine kinases. The ligand binds to receptor type II and will induce the type II receptor to bind to a type I receptor and phosphorylate it. The phosphorylated type I receptor is now activated and will in turn phosphorylate and activate a “receptor-activated SMAD” molecule (R-SMAD) [9]. The R-SMAD represents the intracellular mediator of the ligand and is responsible for transmitting the TGF-ß or BMP signal into the nucleus.
In humans, there are more than 30 different ligands in the TGF-ß superfamily [10]. There are also seven type I receptors and five type II receptors [9]. A ligand-receptor complex can be made by numerous combinations of ligand, type I and type II receptors.
While the type II receptor determines the ligand specificity, the type I receptor determines the SMAD specificity [8]. Ultimately, BMP ligands result in the activation of SMAD1, SMAD5, and SMAD8, while the TGF-ß ligands result in the activation of SMAD2 and SMAD3 [9]. All R-SMAD molecules must then necessarily bind to cytoplasmic SMAD4 molecule to form a functional unit that will in turn enter the nucleus. Once in the nucleus, this SMAD4/R-SMAD functional unit will bind to specific transcription factors and regulate gene expression [9, 10]. Later on we will show that the SMAD-4 molecule, which links both the BMP and the TGF-ß pathways, interacts directly with GATA4, one of the most important regulators of valve formation whose cofactors have been implicated in TA. It is this link between GATA4 and SMAD4 that will tie the BMP and TGF-ß pathways to TA.
Figure 51.2 illustrates the similarities of the TGF-ß and the BMP pathways and their mechanism of action in the heart.
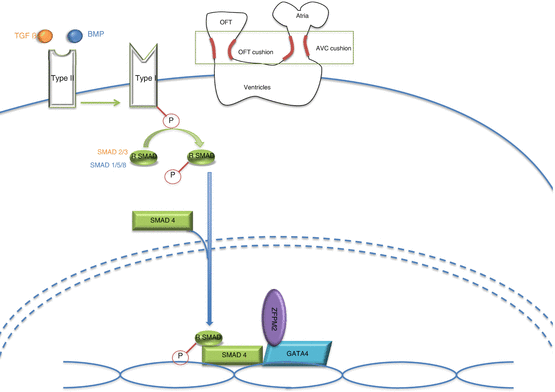

Fig. 51.2
TGF-ß or BMP ligand will bind to the type II receptor that will activate the type I receptor. The type I receptor will phosphorylate a particular R-SMAD molecule (SMAD2/3 for TGF-ß and SMAD 1/5/8 for BMP). This R-SMAD molecule will complex with a SMAD4 molecule to enter into the nucleus and interact with transcription factors such as GATA4
51.3.1.1 Role of TGF-ß Protein in Tricuspid Valve Formation
Although TGF-ß and BMP belong to the same superfamily and share many characteristics, they play distinct roles in tricuspid valve formation. These differences can be seen in the various functions that each pathway plays in EMT.
During embryogenesis, cells that are destined to form valves undergo three separate rounds of EMT, aptly named primary, secondary, and tertiary EMT. Each round of EMT is followed by a round of MET to reset the cells into the epithelial state in preparation for the next round of EMT. Primary EMT results in the formation of cardiac progenitor cells. Secondary EMT plays an important role in the formation of the cardiac tube. Finally, tertiary EMT results in valve formation [11]. TGF-ß and BMP play essential roles in tertiary EMT.
TGF-ß is one of the earliest molecules to be involved in EMT. Studies carried out in mice and chick embryos have shown that TGF-ß acts as an inductive stimulus for EMT, as well as a regulator of later steps in the EMT program. Scientists studied the various isoforms of TGF-ß in the chick embryo and found that TGF-ß2 is essential for EMT initiation, while TGF-ß3 is essential for mesenchymal cell invasion into the ECM [12]. In mice, the function of the TGF-ß genes was slightly different. Studies performed in mice using functional antibodies against different isoforms of TGF-ß concluded that TGF-ß1 and TGF-ß3 were not needed for EMT to occur. TGF-ß2, however, did play a role. In vitro studies showed that the number of mesenchymal cells that invaded the collagen assay upon TGF-ß2 antibody exposure was significantly reduced. Along the same lines, in vivo studies with TGF-ß2−/− mice showed an abnormality in EMT that resulted in hypercellular cushions [13].
Besides the TGF-ß ligand itself, the TGF-ß receptor has an important role in EMT (indicating perhaps that other pathways besides those activated by the TGF-ß ligand are at play). The importance of the TGF-ß receptor has been studied in mice and chick embryos. Studies in chick embryos showed that if TGF-ß receptor type II is blocked, EMT does not take place [6]. In mice, it appears that the TGF-ß ligand-receptor interaction is more complicated. In vivo studies showed that blocking TGF-ß receptor type II does not affect EMT. In vitro studies, on the other hand, showed that blocking the same receptor inhibited explanted tissue EMT. This implies that other compensatory mechanisms, possibly unrelated to the TGF-ß ligand, are able to make up for the loss of the receptor [5].
51.3.1.2 Role of BMP in Tricuspid Valve Formation
Although similar in importance to the TGF-ß pathway discussed above, BMP seems to have a more upstream role than that of TGF-ß in valve formation. Previous reports have shown that BMP2 is important in the initiation of the EMT process. Experiments done on mouse AV canal explants showed that in the absence of the myocardium, endocardial cells exposed to BMP2 are able to undergo EMT. Therefore, BMP2 released from the myocardial cells must be part of the inductive stimuli that kick off EMT in the endocardial cells. Furthermore, BMP2 resulted in an upregulation of TGF-ß in these cells, indicating that perhaps BMP2 is upstream of the TGF-ß pathway in EMT [5, 6]. BMP4 is more important in the process of valve maturation as opposed to EMT initiation [6]. BMP5, BMP6, and BMP7 have been investigated together in the mouse heart. Double knockout mice for BMP5 and BMP7 did not form a cardiac cushion, while double knockout mice for BMP6 and BMP7 showed a delay in the cardiac cushion formation that affected the outflow tract cushion more than it did in the AV cushion [6].
The effect of BMP on EMT can also be appreciated by blocking the BMP receptors. Indeed, blocking the BMP2 type I receptor in mice resulted in mice embryos that failed to undergo EMT and had a decrease TGF-ß expression – similar to BMP2 knockout mice [5].
51.3.1.3 The TGF-ß/BMP Pathway in TA: A Role for ZFPM2 and SMAD
While one can draw distinctions between the roles of TGF-ß and BMP in early valve formation, the fact that they belong to the same superfamily and therefore share a common mechanism of action means that these two pathways will overlap. They do so at the level of the SMAD molecules and more specifically SMAD4. Understanding the role of SMAD4 in both pathways and the role it plays alongside one of the main regulators of valve formation (GATA4) is the key to understanding the roles of TGF-ß and BMP in TA.
The SMAD proteins play a vital role in the downstream pathway of both TGF-ß and BMP. After binding of either BMP or TGF-ß to a surface receptor, a series of events occurs that ultimately results in the activation of a ligand-specific R-SMAD molecule. SMAD4 will then bind to the activated R-SMAD molecule and translocate it into the nucleus [9, 10, 14]. Once in the nucleus, the SMAD4/R-SMAD complex interacts with other transcription factors, including the master regulator of cardiac development, the GATA4 protein. Studies have shown that disruption of this interaction could lead to CHD mainly related to atrioventricular septation and valve formation. In fact, mice double heterozygous for both genes die in utero because of atrioventricular septal defects. Furthermore, in vitro experiments show a strong physical and functional interaction between SMAD4 and GATA4 [15].
In short, the BMP and TGF-ß pathways must signal through SMAD4 to relay their message into the nucleus. SMAD4, in turn, interacts with several transcription factors, including GATA4. The way that all of this ties in with TA is through GATA4’s transcriptional partner ZFPM2.
ZFPM2 (also known as FOG2) is a zinc finger protein with 8 zinc finger domains. It is highly expressed in the heart where it interacts with GATA4 and mediates its activity [16, 17]. ZFPM2−/− mice die in utero between days E13–E14 from a cardiac phenotype that mirrors TA [18].
The molecular pathways that lead to TA are not yet well defined. We have at our disposal only pieces of the puzzle that seem to implicate an intricate relationship between upstream factors like TGF-ß and BMP and downstream targets like GATA4 ZFPM2 and SMAD4. The identification of transcriptional targets for the GATA4/SMAD4 and GATA4/ZFPM2 interactions would help establish a better genotype/phenotype correlation and a comprehensive mechanism that links the transcriptional outcome activities with the underlying defect.
51.4 The Notch-Hey2 Pathway
Besides the BMP and the TGF-ß pathways, the Notch pathway is one of the upstream regulators of EMT and valve formation [5, 6]. Not unlike the previous two pathways, the Notch pathway is vital for several cellular processes including differentiation and patterning. It distinguishes itself from the TGF-ß and the BMP pathways, however, by using ligands that are not secreted. The Notch pathway involves a membrane-bound receptor binding to a neighboring cell’s membrane-bound ligand [19]. This makes the Notch signaling pathway contact dependent, unlike either the BMP or the TGF-ß signaling pathways.
There are four Notch receptors in mammals and they all share the same basic structure. An extracellular domain will bind to one of the five Notch ligands (Dll 1, Dll 3, and Dll 4 and Jag 1, Jag 2), an intracellular domain (NICD) that detaches from the receptor and moves into the nucleus, and a transmembrane domain [19, 20].
After the Notch binds to its ligand, a series of reactions ensue that ultimately result in the detachment of the NICD and its translocation into the nucleus. Once in the nucleus, the NICD forms a complex with the RBPJK (Recombinant signal Binding Protein for immunoglobulin kappa J region) transcription factor. This NICD/RBPJK complex recruits the co-activator protein MAML (MAsterMind-Like), and this entire complex upregulates the expression of several downstream targets, including Hey2 and SNAIL. Figure 51.3 illustrates this contact-dependent Notch signaling pathway.
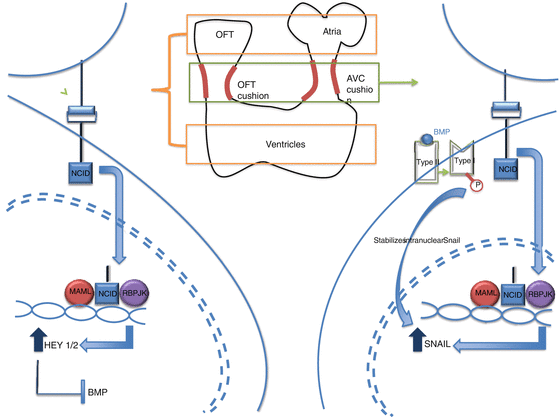

Fig. 51.3
After Notch binds to its ligand in a cell contact-dependent manner, it will release its NCID domain. This domain will travel into the nucleus and form a complex with MAML and RBPJK and upregulate different factors depending on what area of the heart this is taking place in. In the atria and ventricle (right side of the figure), the Notch pathway will result in the upregulation of HEY1/2 that will in turn block BMP signaling. This will work to inhibit EMT. In the cushion regions (left side of the figure), the Notch pathway results in the activation of SNAIL and will induce EMT. BMP will stabilize the SNAIL in the nucleus
51.4.1 Role of Hey2 Notch in Tricuspid Formation and TA
Like TGF-ß and BMP, Notch also plays an important role in normal valve formation. The importance of the Notch pathway in normal tricuspid valve formation was realized in studies that showed severely dysmorphic cardiac cushions upon inhibition of Notch in mice. Upregulation of Notch, on the other hand, results in hypercellular cushions [6]. Detailed spatial and temporal analysis of the expression of Notch during cardiac cushion development reveals an important role for Notch in the regulation of the EMT process in the tricuspid valves. Analysis of the spatial distribution of the Notch ligand, Dll4, just prior to EMT reveals its presence in high amounts in areas destined to become valves [21]. Predictably, Notch itself also was present in these atrioventricular canal (AVC) areas prior to EMT initiation. Furthermore, inhibition of Notch, either directly or by inhibiting its RBPJK transcription factor, resulted in endocardial cells that are unable to migrate into the cardiac jelly [19, 21], which indicates that Notch must play a role in EMT and tricuspid valve formation.
Besides allowing EMT to occur, the Notch signaling pathway is also important for patterning the premature cardiac cushion areas. It seems that some of the transcription factors activated downstream of Notch, specifically Hey1 and Hey2, determine which areas will undergo EMT by inhibiting essential EMT signals in the tissue in which they are present. Just prior to valve development in the mouse, Hey1 is present in the atrial myocardium and endocardium, and Hey2 is present in the ventricular endocardium. Thus, these downstream Notch cofactors inhibit EMT in the ventricle and atria and allow EMT to proceed only in the AVC and the OFT cushion regions where they are not present [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


