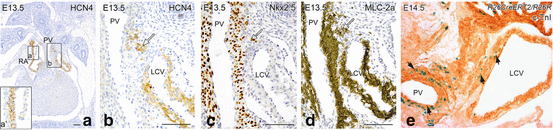
Fig. 30.1
Schematic representation of subsequent stages of cardiac development. (a) The venous pole of the heart encompasses the sinus venosus (SV) and the atrium (A). Initially, the SV consists of, more or less symmetrical, left and right horns that receive blood from the common cardinal vein (VCC), the left and right vitellin veins (VVS and VVD, resp.) and the left and right umbilical veins (VUS and VUD, resp.). (b, c) Due to an asymmetrical expansion of the common atrium, a rightward shift of the connection of the sinus to the atrium occurs. The right-sided cardinal vein will form the superior caval vein (VCS). The left side in human will largely obliterate, the remaining part will form the coronary sinus (CS) and the vein of Marshall (OVM, remant of the left cardinal vein). Initially, the single pulmonary vein Anlage will drain rather central in the atrium. After septation, the pulmonary veins (PV) will drain into the left atrium. GCV great cardiac vein, LV left ventricle, OFT outflow tract, OVM oblique vein of Marshall, RV right ventricle, V common ventricle, VCI inferior caval vein (Source: [41])
The inner lining of the myocardial tube consists of endocardial cells that are continuous with the endothelium of the blood vessels. As a consequence of left–right patterning, the heart tube is asymmetric and loops to the right in normal development. Major parts of both the arterial and venous pole are derived from the splanchnic mesoderm behind the heart during development, referred to as contributions from the second heart field (SHF). The primitive heart tube thus expands by intrinsic growth patterns of the cardiac tube, but also by extrinsic additions at both the arterial and the venous pole at expense of the SHF splanchnic mesoderm, as shown by expression patterns of, e.g. Tbx (T-box) 5, GATA (GATA binding protein) 4–6 and NKX2-5 (NK2 homeobox 5) [3], Islet1 (ISL LIM homeobox 1) [4] and Id2 (inhibitor of DNA binding 2) [5]. During heart development, cardiac chambers and transitional zones appear, the latter involved in septation, valvulogenesis and formation of the conduction system. The future atrial and ventricular components are separated by the atrioventricular canal. At the venous pole, recruitment of myocardium has been reported to depend on Pitx2c (paired-like homeodomain 2), Nkx2-5, Tbx18, Shox2 (short-stature homeobox 2) and Pdpn (podoplanin) [6–10]. This region located posterior to the primary heart tube, as part of the SHF is referred to as the posterior second heart field being involved in addition of myocardium to the sinus venosus, the pulmonary veins, the cardinal veins, as well as to the body of the atria.
30.2 Pulmonary Plexus and Vein Development
During the 4th week of embryonic human development, the primary lung buds appear at the apex of the endodermal respiratory diverticulum, surrounded by splanchnic mesoderm (Fig, 30.2). The lung buds differentiate into the bronchial system and their bifurcations in the future lungs. The splanchnic mesoderm provides the pulmonary vascular smooth muscle and connective tissues as well as the capillaries, the latter developing into a splanchnic plexus by which the primitive lungs drain into the systemic circulation. The splanchnic plexus is connected to the venous pole of the primitive heart tube by a strand of endothelial cells, the so-called mid-pharyngeal endothelial strand that becomes the common pulmonary vein (Fig. 30.2a, g). The MPES is initially not lumenized, and the main route of drainage to the venous pole of the heart is via connections of the pulmonary splanchnic plexus to the embryonic systemic veins (cardinal, umbilical and vitellin veins). This early drainage pattern has also been referred to as a peripheral drainage pattern. Upon lumenization of the MPES, the lungs initially drain both centrally to the atrium and peripherally to the systemic veins, the so-called intermediate drainage period (Fig. 30.2b). After atrial septation, the pulmonary–systemic connections are lost and regress (Fig. 30.2c), the beginning of the central drainage period [11] (Fig. 30.2d–g). Persistence of early pulmonary-to-systemic connections is likely the substrate for anomalous pulmonary venous connections. Several forms of anomalous pulmonary venous connections can be attributed to a deficiency in development towards the establishment of a central drainage pattern (Table 30.1).
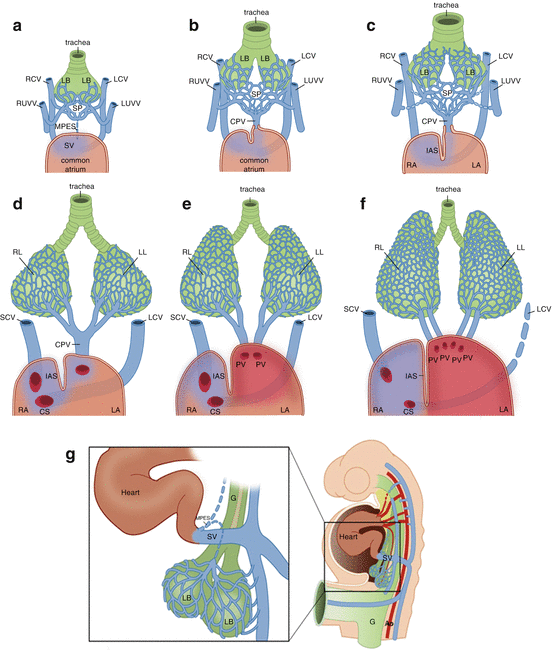

Fig. 30.2
Schematic overview of pulmonary vein development, frontal views. (a) Peripheral draining period. During the 4th week of embryonic development, 2 lung buds (LB) can be discriminated at the caudal end of the respiratory diverticulum of the foregut. These lung buds into the bronchi and their bifurcations in the future lungs. The splanchnic mesenchyme forms a splanchnic plexus (SP) of veins that is already connected to the endocardium of the primitive heart tube, by means of a strand of endothelial cells, the mid-pharyngeal endothelial strand (MPES), the Anlage of the common pulmonary vein. Initially this strand is not lumenized yet; during early embryology, the normal route of drainage is from the pulmonary venous plexus to the systemic veins via pulmonary-to-systemic venous connections. (b) Intermediate draining period. At this stage, the MPES has lumenized to become the common pulmonary vein (CPV), allowing drainage of the splanchnic plexus not only to the systemic veins but also into heart. (c) As the CPV grows and dilates and becomes the main route of drainage of the pulmonary venous blood, the primitive pulmonary-to-systemic connections will gradually regress. (d) Central drainage period. During normal development, the primitive pulmonary-to-venous connections will regress completely, and the only route of drainage of the pulmonary venous blood is now directly into the heart. (e, f) During further development, bifurcations of the CPV will be incorporated into the left atrium (LA), usually to an extent that four separate pulmonary venous ostia (2 right sided and 2 left sided) can be distinguished, although variations occur [17]. The right cardinal vein (RCV) has become the superior caval vein (SCV), and the left cardinal vein (LCV) will partly become the coronary sinus (CS), whereas the extracardiac part of the LCV will regress to become the ligament of Marshall. (g) Lateral view of the embryo with a magnification of the boxed area. A splanchnic vascular network surrounds the lung buds (LB) that grow out from the foregut (G). G foregut, IAS interatrial septum, LB lung buds, LL left lung, LUVV left umbilical and vitellin veins, RA right atrium, RL right lung, RUVV right umbilical and vitellin veins, SV sinus venosus
Table 30.1
Abnormal pulmonary vein development in relation to anatomical level, time and clinical entities
Anatomical level | Anomaly | Developmental impairment | Time relation (in relation to atrial septation) | Pulmonary-to-systemic connections | Clinical entity |
|---|---|---|---|---|---|
Common PV/MPES | Absence | Absent connection PV-LA (absent MPES) | Before | Will persist | TAPVC (extracardiac type) |
Atresia | Non-lumenization of the MPES (PV Anlage) | Before | Will persist | TAPVC (extracardiac type) | |
Stenosis | Secondary stenosis of an initially lumenized common PV | Before/during | Will disappear | Cor triatriatum | |
Atresia | Secondary obliteration of an initially lumenized PV | After | Have disappeared | TAPVC (lethal) | |
First or second PV bifurcation | Atresia | Non-lumenization of 1 or more individual PV(s)/tributaries | During | Will persist | PAPVC (extracardiac type) |
Stenosis | Secondary stenosis of an initially lumenized individual PV | Before/during After | Will disappear Have disappeared | Congenital PV stenosis Acquired PV stenosis | |
Atresia | Obliteration of 1 or more initially lumenized PVs | After | Have disappeared | (Solitary) PV atresia | |
Veno-atrial junction | Variant number of PVs | Abnormal PV incorporation | After | Have disappeared | Unilateral common |
<4 | Incomplete | PV ostiuma | |||
>4 | Extreme | >4 PV ostiaa | |||
Dorsal mesenchymal protrusion | Hypoplasia | Abnormal mesenchymal contribution from SHF | During | Will disappear | Sinus venosus defect with PAPVC (cardiac type) A(V)SD |
Absence | Absent mesenchymal contribution from SHF | During | Will mostly disappear | TAPVC (cardiac type) A(V)SD |
30.3 Myocardialization of the Pulmonary Veins
The entrance of the common pulmonary vein to the sinus venosus is bordered by two muscular ridges (Fig. 30.3). The right-sided pulmonary ridge merges with the so-called dorsal mesenchymal protrusion (DMP), important for formation of the base of the atrial septum [12, 13]. As a consequence, the pulmonary vein between the two muscularized ridges will at the left side be incorporated in the posterior wall of the left atrium, thereby contributing to the histological characteristics of the left atrial body, consisting partly of vascular smooth muscle cells and partly of myocardium [14]. The DMP is derived from the embryonic second heart field that comprises an area of mesenchyme behind the heart that will contribute to the embryonic heart during further development [3].
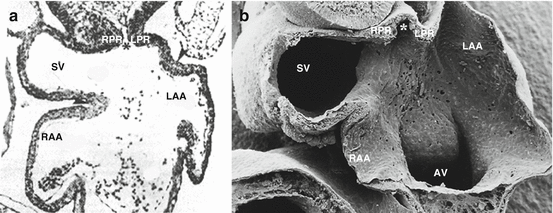

Fig. 30.3
Transverse light microscopic section (a) and electron microscopic (b) section at the level of the sinus venosus (SV) and atrial appendages (RAA and LAA) of a chick embryo. The entrance of the common pulmonary vein (asterisk) to the SV is bordered by two muscular ridges, the left (LPR) and right pulmonary ridge (RPR). The latter merges with the so-called dorsal mesenchymal protrusion (not shown), at the base of the interatrial septum, causing the pulmonary vein to be located at the left side after atrial septation (Source: Panel a adapted from [42])
The common pulmonary vein becomes myocardialized and gives rise to separate venous orifices in the posterior left atrial wall. The degree of incorporation is variable, and not all individuals have four distinct pulmonary venous ostia, while common ostia of particularly the left pulmonary veins as well as additional pulmonary venous ostia are often observed [15–17].
The wall of the pulmonary veins will develop a myocardial sleeve as is the case with the cardinal (caval) veins. The myocardial continuity of the atrial and venous walls, varying in different species, has been thoroughly investigated by several groups and has led to different opinions regarding the origin and differentiation of the involved cell populations [7, 14, 18–20].
30.4 Histological Characterization During Normal Development
Pulmonary vein development in human embryos takes place between 7 and 22 weeks (crown rump length 19–170 mm). Using various specific antibodies for muscle actins, alpha-smooth muscle actin and atrial myosin light chain, three different areas can be distinguished in the posterior left atrial wall: (1) the smooth-walled atrial body with incorporated pulmonary vein smooth muscle cells, (2) the trabeculated appendage without vascular smooth muscle cells and (3) the transitional zone between body and appendage (see Fig. 19.2). The transitional zone histologically resembles the sinus venosus and presents with a very thin and even absent myocardium, from which it was hypothesized that during incorporation of the pulmonary vein into the body of the left atrium, the area of the sinus venosus myocardium was reduced to the narrow zone encircling the entrance to the atrial appendage [14]. These observations are relevant for interpreting the definition of the sinoatrial transition and the sinus venosus, but also for the interpretation of areas of preferential induction of arrhythmias in later life. Interestingly, the body of the right atrium, the right atrial appendage and the right-sided face of the atrial septum are not lined by vascular smooth muscle cells.
30.5 Origin and Incorporation into the Left Atrium
The origin and incorporation of myocardium and smooth muscle cells into the pulmonary vein and left atrium has been investigated using various approaches. Based on overlapping gene expression patterns in the SHF of Pitx2c, Islet1, Tbx18 and Nkx2-5, clues are provided to the origin of myocardial cells enveloping the caval and pulmonary veins together with segments of the atrium (elegantly summarized by Lescroart et al. [19]). The topic of connection of the pulmonary vein to the sinus venosus and subsequent incorporation into the left atrium is marked by a fierce debate [21–25], but it is unnecessary to repeat the various arguments here. It suffices to note that myocardium associated with the left-sided veins (pulmonary vein and left cardinal vein, including the dorsal left atrium) shares a common lineage, as does myocardium associated with the right-sided veins including the dorsal right atrium [19]. Therefore, we favour an original left-sided connection of the common pulmonary vein to that part of the sinus venosus that will be incorporated in the dorsal wall of the left atrium. With this concept in mind, we must realize that patterning of the various developmental players (second heart field, DMP, myocardium, smooth muscle cells) will be reflected by the (epi)genetic regulation networks and in case of TAPVC by misregulation.
It is evident that different areas of gene expression can be recognized, as Pitx2c is expressed in the myocardium of the pulmonary vein, left superior caval vein and (left) part of the atrium, Nkx2-5 in the pulmonary vein myocardium/mesenchyme and atrial myocardium, Tbx18 only in the myocardium surrounding the caval veins and Nppa (natriuretic peptide A) only in the atrial wall. Podoplanin is expressed in the myocardium and smooth muscle cells of the pulmonary veins, as well as in the left atrial dorsal wall including the left and right venous valves. The temporospatial expression pattern of these genes in SHF progenitors based on the use of reporters and transgenes in mice is intricate [7, 9, 10, 26]. A recently published approach initiated by Buckingham et al. [27] exploits retrospective clonal analysis based on rare random events of recombination of a nonfunctional nLaacz sequence into functional nLacZ, targeted to the alpha cardiac actin gene [19], the recombination event being independent of gene expression. They conclude that there are left and right myocardial sublineages at the venous pole of the heart. The pulmonary and left superior caval vein share common progenitors with dorsal left atrial myocardium (Fig. 30.4), whereas the right superior caval vein and dorsal right atrial myocardium share their own common progenitors, illustrating the difference between genetic tracing experiments and lineage analysis. Furthermore, these results suggest that the caval vein myocardium does not have a separate clonal origin from the pulmonary myocardium.