Mitral Valve Repair for Rheumatic Disease
Arkalgud Sampath Kumar
Introduction
Acute rheumatic carditis produces valvulitis of all four valves, in addition to pericarditis and myocarditis. It is most often seen in children, who present with gross CHF, arrhythmia, mitral, tricuspid and aortic regurgitation. It is best to treat these children conservatively with decongestive therapy, arrhythmia control, aspirin, and steroids. Surgery carries a high risk of mortality and failure.
Chronic RHD affects the MV more often than the AV and TV. Involvement of all four valves is not uncommon. MS with or without MR is the usual manifestation.
MS; pure, noncalcific, isolated MS in normal sinus rhythm can undergo balloon mitral valvotomy (BMV) or CMV. Patients with AF, intracardiac thrombus, calcification, or restenosis will require (OMC). The techniques for closed and open mitral commissurotomy and repair for pure MS are described in Chapter 19. This chapter will focus on MV repair for rheumatic MR and for MS with regurgitation. Patients with combined MS and MR or with pure MR require correction under direct vision. MV repair is the operation of choice. With experience, nearly 70% of patients with MR can undergo successful repair with good long-term results.
Before heparinisation a complete TEE is performed to assess:
MV cusps; thickness, mobility, fusion, and calcification. The anterior mitral leaflet (AML) length from annulus to free edge in the four-chamber view at the P2 level is measured. If this length is 26 mm (18 mm/m2 body surface area) then the likelihood of successful repair is high.
The presence of LA/left atrial appendage (LAA) thrombus, LA size, MS prolapse, and subvalvular apparatus are also thoroughly assessed.
The TV and AV need to be carefully assessed. However, a decision to do TV repair is best taken on the basis of TTE performed before surgery. Assessment of TR can be fallacious under anesthesia. If the tricuspid annulus diameter exceeds 40 mm and TR
is moderate or severe, then TV repair will be necessary. TS is commonly seen in RHD and will always require correction.
is moderate or severe, then TV repair will be necessary. TS is commonly seen in RHD and will always require correction.
Median Sternotomy
This is the standard approach for most open heart procedures. For cosmetic purposes the incision in the skin can be made from the sternal angle to xiphoid, especially in young women. The sternum is divided in a lazy S-shaped incision (interlocking sternotomy, Fig. 20.1). This provides better healing, less pain, and improved postoperative respiratory function. A 4 inch piece of sterile plastic tubing(venous line) is split longitudinally and placed around the sternal edge to protect the sternum on both sides.
The pericardium is opened in the midline for routine exposure of the heart. In a redo sternotomy, care must be taken to separate the heart from the back of the sternum before using a retractor. Pericardial stay sutures are placed to expose the aorta and the right chambers. The left pericardial flap is not pulled up as it may hamper MV exposure. The heart may be adherent to the pericardium and needs to be completely freed. This helps to get better exposure, allows proper surface cooling, and complete de-airing.
Right Thoracotomy: For better cosmesis especially in young females a right thoracotomy can be used for MV repair (see Chapter 19 for details).
Cardiopulmonary Bypass
For MV and TV surgery the technique used is warm body, cold heart. This technique has several advantages.
It saves time (cooling/rewarming)
Reduces coagulation abnormalities
Reduces inflammatory reaction to CPB by reducing CPB time
The SVC and IVC are cannulated through the appendage and body of the RA. If the RA is to be opened, then the SVC and IVC are taped. The ascending aorta is cannulated at its highest point. An aortic vent cannula to deliver cardioplegia and to de-air is placed on the
highest point of the ascending aorta. It must be placed with sufficient space between it and the aortic cross clamp to clamp the aorta or to work on the AV if necessary.
highest point of the ascending aorta. It must be placed with sufficient space between it and the aortic cross clamp to clamp the aorta or to work on the AV if necessary.
Myocardial Protection
Cold blood cardioplegia is the preferred method. It is delivered into the ascending aorta after clamping. The dose is 15 mL/kg body weight. This is combined with topical ice slush cooling of the heart externally. In patients with AV disease the cardioplegia solution is delivered antegrade into the coronary ostia, 70% into the left coronary artery (LCA), and 30% to the right coronary artery (RCA). Cardioplegia is repeated every 20 to 25 minutes. The last dose of cardioplegic solution is administered at a temperature of 32º to 34ºC. This effectively rewarms the heart and spontaneous cardiac rhythm returns when the aortic clamp is released. Defibrillation is rarely necessary.
Blood Conservation
It is important to avoid homologous blood transfusion when possible. In primary valve surgery, nearly 80% of patients can undergo safe surgery without blood/blood product transfusion.
Autologous donation of 300 to 500 mL of blood is an extremely useful method in adults. It must be done cautiously. In patients with a hemoglobin level of 12 g% or more and 40 kg body weight this can be completed before heparinization.
The CPB circuit is primed with crystalloid solution. A minimum quantity is retained, discarding the excess before beginning cannulation. If the patient’s blood pressure permits, the aortic cannula is drained retrograde into the oxygenator while discarding the crystalloid prime (retrograde priming). This way CPB can be conducted with a blood prime (higher viscosity) reducing hemodilution. A pump flow of 70 to 80 mL/kg body weight is maintained during perfusion. The systemic perfusion pressure should be maintained above 50 mm Hg throughout perfusion.
Exposure of the Mitral Valve
If the LA is enlarged, a generous incision behind the Waterston groove provides the best exposure. In patients with an atrial septal defect (ASD) or requiring a TV repair, the MV may be approached through a transseptal incision.
Assessment
As soon as the cardioplegic solution is delivered the LA incision is enlarged. A Cooley mitral retractor is placed and the assistant retracts the atrial septum. The LA cavity is inspected for (a) thrombus and (b) McCallum patch (jet lesion). The jet lesion is a sign of prolapse and is usually found behind the PML in patients with AML prolapse. Any thrombus is completely removed and the LA is thoroughly irrigated. The thickened endocardium of the jet lesion is peeled off (it can be a source of thrombus formation). An LA vent is now placed in the left inferior pulmonary vein and connected to the vent line.
Two hooks are placed through the mitral orifice—one behind the AML and another behind the PML. If the leaflets are not thickened, the tip of the hook can be easily seen through the leaflet tissue. The commissural fusion lines easily identify MS if present. Look for calcification, especially at the commissures and any soft thrombus. An open commissurotomy needs to be performed before further assessment can be done. With the hooks retracting the cusps, an incision is made with the tip of a no. 11 blade just inside (2 to 3 mm) from the mitral annulus at the commissures (see videos).
A fine right-angled forcep is inserted through this incision to separate the cusps from the subvalvular fusion. The commissural fusion line is now gently incised separating the two cusps. Fused chordae are separated by incising with a no. 11 blade. A fused papillary muscle is divided with sharp Potts scissors down to its base allowing the LV cavities come into view.
If there is no MS, the posterior hook pulls up the P1 segment of the PML. This will be the reference point. Any prolapse of the A1/A2/A3 or P2/P3 segments is assessed. Chordal elongation and/or rupture are identified. Ruptured chordae are excised. Saline is injected into the LV to assess prolapse and mobility of the cusps.
Cusp Thinning
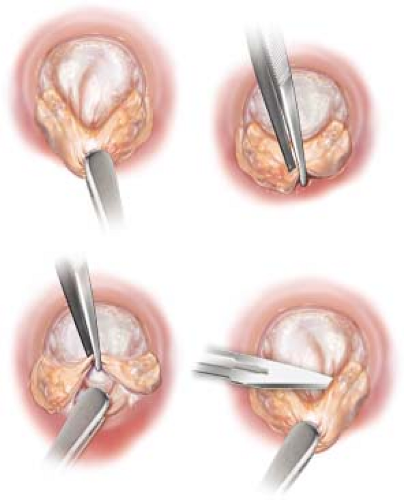
This procedure is extremely useful in RHD. It releases the normal imprisoned cusps, makes them thin and improves mobility, enlarges the cusps, and increases coaptation. It is especially useful in the AML (see video and Fig. 20.2).
The belly of the AML is grasped with a Russian forceps. With the Debakey tissue forceps, the fibrous peel is grasped firmly at the annulus and with a downward traction it is separated from the normal AML which appears glistening (see video). This is now peeled toward the free edge of AML all around. The resultant peel is excised at the free edge.
A similar procedure is performed for the PML also. The thickened endocardial peel is grasped just behind the annulus and peeled toward the free edge. Care must be taken not to tear the cusps. It is then excised at the free edge.
Fenestration
Fused chordae from the free edge to the papillary muscle need to be freed. A right-angled clamp is placed at the tip of the papillary muscle and held by an assistant. Alternately, a hook is placed behind the thickened chordae. Using a no. 11 blade the thick fibrocalcific material is shaved carefully until the chordae become visible. The fusion between the chordae is also incised with the knife to separate them. This improves the mobility of the cusp and abolishes a secondary orifice.
The valve is now assessed by injecting saline into the ventricle. It may be necessary to grasp the posterior annulus and to push it forward during a saline test. With the LV filled, gentle pressure from the outside by gently squeezing the LV will show cusp movement and a residual leak if present.
Acquired clefts between the P1-P2 and P2-P3 scallops may need to be closed using a 5-0 Prolene horizontal mattress suture.
Chordal Shortening
Any prolapse of the AML that is identified can be corrected by shortening the elongated chordae (Fig. 20.3




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree