Fig. 9.1
Anatomy – anterior and posterior leaflets of the mitral valve
The mitral annulus represents the junction that joins the left atrium and ventricle and consists of fibrous and muscular tissues. The average human mitral annular cross-sectional area is 5–11 cm2. During systole, the annulus assumes an elliptical shape and is able to contract and decrease in diameter, whereas, in diastole, it assumes a more circular shape. Annular flexibility allows for increased leaflet coaptation during systole and increased annular orifice area during diastole. The anterior aspect of the annulus, which is composed of the fibrous skeleton of the heart and consists of rigid but elastic fibrous tissues, has limited flexibility, whereas the posterior aspect of the annulus, which is in continuity with the fibrous skeleton and consists of a mixture of muscular and gradually tapering fibrous tissues, contributes most of the annular flexibility.
The chordae tendinae are comprised of fibrous connective tissue chords and attach the leaflets to either the papillary muscles or the LV wall directly. The chordae are divided into three groups. The primary chordae attach directly to the free edge of the leaflet, and ensure that the leaflets coapt without prolapse or flail. The secondary chordae, which are more prominent on the anterior leaflet, attach to the leaflet along the line of coaptation, and are important in maintenance of ventricular function [14]. Tertiary chordae are only present on the posterior leaflet, and attach directly to the ventricular wall or to the trabeculae carnae. In addition, there are commissural chordae, which arise directly from either of the papillary muscles and attach to both leaflets.
The anterolateral and posteromedial papillary muscles project into ventricular cavity directly from the apical and mid portion of the ventricular wall, and give rise to chordae tendinae that attach to both leaflets. The anterolateral papillary muscle receives a dual blood supply from the left anterior descending and from either a diagonal or marginal branch of the circumflex artery. In contrast, the posteromedial papillary muscle has a single blood supply, either from the right coronary or the circumflex artery. Therefore, the posteromedial aspect of the LV wall and the papillary muscle is more susceptible to ischemia and infarction and together play a very important role in valvular incompetence and leaflet malcoaptation in the instance of ischemic MR with a variable degree of posterior myocardial infarction.
The most important determinant of mitral valve competency centers on the zone of coaptation. Mitral valve leaflets accommodate high systolic LV pressure and establish competence through the distribution of force that can be likened to the stresses in a Roman arch in order to lessen the stress to the other parts of the ventricle and mitral valve apparatus. To maintain mitral valve competency in a systolic phase, the adequate zone of coaptation needs to be established in a coordinated fashion. To achieve adequate coaptation, both anterior and posterior leaflets should:
1.
Be close enough to each other
2.
Have enough tissue to cover the length of the zone of coaptation
3.
Be guided at the line of coaptation by the leaflet chords at appropriate angle
Pathophysiology of MR
Acute MR vs Chronic MR
In MR, the regurgitant volume that is ejected into the left atrium is dependent upon regurgitant orifice size, ventricular to atrial pressure gradient, atrial compliance and heart rate. The degree of increase in left atrial pressure, which is associated with congestive symptoms. is significantly affected by the regurgitant volume and the compliance of the left atrium.
The compliance of the left atrium is very different in acute and chronic MR, so it is important to fully recognize and differentiate the chronicity of MR in each patient being evaluated with heart failure.
Acute MR
Causes of acute MR include chordal rupture, endocarditis, blunt chest trauma, or myocardial infarction. The left atrium is of normal size with low compliance. A relatively small amount of acute MR can lead to an acute increase in left atrial pressure and lead to significant pulmonary edema that requires acute treatment. In this setting, symptoms and signs, along with the hemodynamic state dictate the proper timing of surgery. These are patients who benefit from mechanical support of the circulation peri-operatively, either with intra-aortic balloon pump (IABP) counterpulsation or extra-corporeal membrane oxygenation (ECMO).
Chronic MR
In chronic MR, there is a gradual increase in regurgitant flow into the left atrium that leads to atrial enlargement and a significant increase in left atrial and pulmonary venous compliance. Therefore, signs and symptoms of pulmonary congestion may not become apparent until much later in the process of the disease in spite of the significant degree of MR and the significant volume overload and pathologic changes in the LV. In this setting, less symptomatic patients are difficult to triage in terms of proper timing of the intervention [15]. In this review, we treat geometric MR, which is chronic MR due to a distortion of the ventricular geometry. This is considered and treated as a different disease entity from acute MR.
Valvular MR vs Geometric MR
To understand the mechanism and the rational treatment of chronic MR, it is very useful to classify MR into primary/anatomic/valvular MR and secondary/functional/geometric MR.
In valvular MR, regurgitation is caused by structural valvular disease. The etiology of structural mitral valve diseases include degenerative (FED or Fibro-Elastic Degeneration, myxomatous disease, Barlow syndrome and fibroelastosis such as Marfan syndrome and connective tissue diseases), rheumatic, endocarditis, trauma, tumor, inflammatory and congenital. In this setting, problems with the components of the mitral valve apparatus cause MR.
Mitral valve repair of this valvular MR is systematically guided by three functional anatomic categories of mitral valve pathology proposed by Dr. Carpentier in 1983 [16];
Annular dilatation
Leaflet prolapse with elongated or ruptured chordae
Leaflet restriction
The treatment of valvular MR aims to establish a zone of coaptation according to the functional anatomy. Mitral valve repair techniques in this setting include annuloplasty, variable degrees of leaflet resection, advancement or “sliding” plasty, chordal transplantation, and PTFE neo-chordal implantation.
In contrast, secondary or functional MR is defined as MR that is not caused by a structural defect of the components of the mitral valve apparatus but rather is caused by a distorted functional position of the components of the mitral valve apparatus related to LV dilatation. Therefore, functional MR is not a valvular disease but a geometric ventricular disease. Often in this setting, the dilatation of the ventricle may be secondary to an ischemic etiology with inferobasal akinesis or dyskinesis contributing to the MR. Figure 9.2 shows how inferobasal scarring and dyskinesis can contribute to mitral regurgitation. Figure 9.3 shows the stresses placed on the mitral valve leaflets through the connection of the chordae to the ventricular muscle, suggesting that functional MR is a disease of the ventricle. Figure 9.4 shows that scarring of the muscle around insertion of the posteromedial papillary muscle.
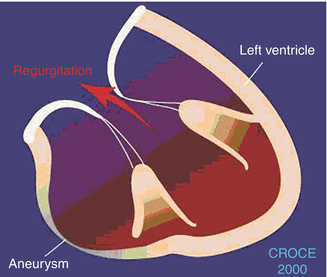
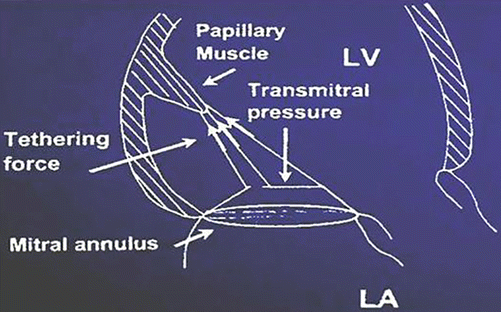
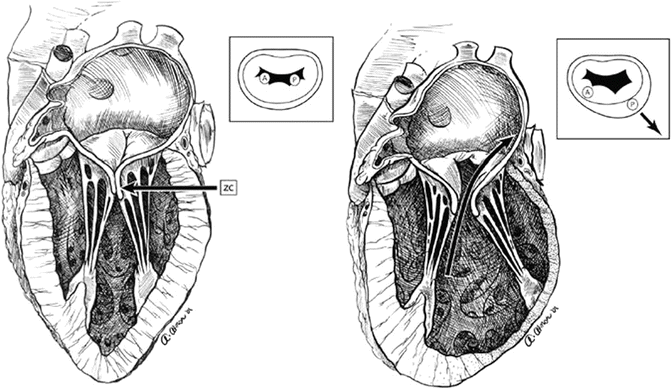

Fig. 9.2
Mechanism of MR with inferobasal scarring of the LV

Fig. 9.3
Stresses on mitral valve leaflets and chordae attached to the papillary muscle

Fig. 9.4
Perturbation of coaptation of the mitral valve leaflets, due to scarring of left ventricle affecting the posterobasal papillary muscle, causing eccentric MR
DCM is defined by clinical evidence of chronic and progressive heart failure associated with echocardiographic findings of poor cardiac contractility (reduced LV systolic function reflected in reduced LV ejection fraction) and ventricular dilatation.
According to the primary etiology, DCM is usually classified into ischemic DCM and non-ischemic DCM because ischemic DCM is most prevalent.
Non-ischemic DCM can further be classified into idiopathic DCM (ventricular etiology) and valvular DCM (valvular etiology). Figure 9.5 shows the displaced papillary muscles causing central mitral regurgitation. It should be noted that there is a segment of this patient population that have concurrent ischemic heart disease and MR from degenerative mitral valve disease at the same time, commonly seen in elderly patients. This group is distinct from patients with geometric MR with ischemic DCM.
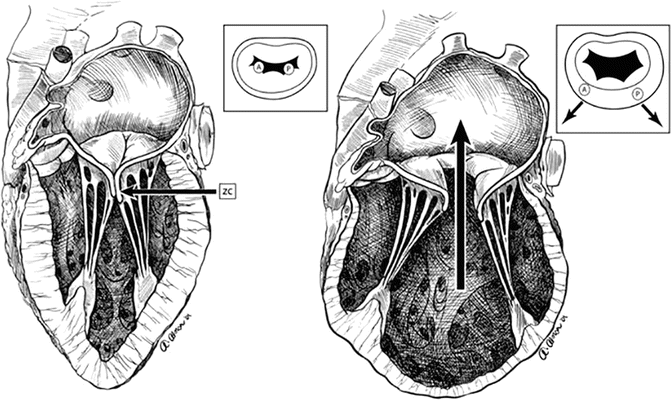

Fig. 9.5
Loss of coaptation of the mitral valve leaflets, due to displacement of both papillary muscles in dilated cardiomyopathy, causing central MR
In geometric MR the components of the mitral valve apparatus itself are normal, two of the three functional anatomic categories proposed by Dr. Carpentier hold true;
Annular dilatation, which may be mild or moderate
Leaflet restriction: papillary muscle – LV wall displacement
Figure 9.3 shows the components of geometric MR. The treatment aim of geometric MR is to re-establish the zone of coaptation to eliminate regurgitation. To achieve this goal, a flexible complete ring annuloplasty technique was initially used [17]. As surgeons became more comfortable with this technique, more and more aggressive undersizing and overcorrecting ring annuloplasty was used based on the assumption that the most significant determinant of leaflet coaptation in geometric MR is the diameter of the mitral valve annulus and that undersizing/overcorrecting the annulus would also help to compensate the widened angle and the septolateral distance of the papillary muscles resulted from LV dilatation. These clinical observations and acceptable surgical results were correlated with several key echo parameters as well as improvement of symptom and Quality of Life [18–20].
The natural history of chronic degenerative or rheumatic valvular MR can be protracted and long. The progression of the disease may be slow and span several decades. The onset of symptoms are often masked and may present late in the progress of the disease even with elevated left atrial and LV end-diastolic pressures because of a large compliant left atrium and the slow, gradual progression of this process. The presence of MR contributes to this increased back pressure and a decreased forward flow as well. Decreased forward flow is also well compensated with increased total stroke volume that is a combination of regurgitant volume plus effective forward stroke volume and increased heart rate. This leads to increased workload from volume overload to the LV. Short-term or medium-term neurohormonal compensatory mechanism also play a role in maintaining the same effective forward stroke volume in the setting of chronic MR. The normal LV can accommodate a fairly large amount of regurgitant volume and be well compensated before the LV starts to dilate. This is reflected in hyperdynamic wall motion and increased LV ejection fraction. However, once increased volume overload reaches beyond the compensatory adaptation of the LV, the LV is forced to dilate through homeostatic compensation mechanisms according to the Frank-Starling curve. This is reflected in an increased LV end–diastolic and end-systolic volume or dimension producing chronic LV dilatation and increased LV wall tension and stress. The LV reacts by trying to match the increased wall tension/stretch/stress by proportionally increasing LV mass via LV hypertrophy. As LV dilatation progresses, the LV wall tension/stretch/stress increases and coronary flow reserve decreases in addition to increased LV work load by volume overload [20–23]. Increased LV wall stress is the most potent stimulus for progressive heart failure through the mechanism of LV remodeling at a genetic, molecular and neurohormonal level. This chronic energy imbalance of increased workload plus increased wall tension/stretch/stress and decreased coronary flow reserve accelerates the damage to the LV muscle through the loss of LV contractile functional reserve. The poor survival of patients with chronic degenerative MR has been well established. Even though total resistance to the LV is decreased by MR, the total workload to produce the same forward flow is proportionally increased to volume overload with MR. The development of clinical symptoms usually reflects significant LV dysfunction and is a hallmark of reduced life expectancy. In the current setting of low operative mortality and high feasibility of repair, the timing of mitral valve repair can be offered at earlier stages of the disease in hopes of changing the natural history of the disease and before the development of left ventricular dysfunction [15]. A regurgitant volume of ≥60 ml/beat and effective regurgitant orifice area of ≥40 mm2 are currently recommended as an index of quantitative assessment for the timing of mitral valve repair in asymptomatic patients with degenerative valvular MR [24, 25].
Geometric MR with DCM further contributes to LV dilatation and remodeling through chronic phenotypical changes triggered by increased volume work-load, increased LV wall tension/stretch/stress and decreased coronary flow reserve in addition to originally dysfunctional LV. This LV dilatation and spherical change further increases the magnitude of MR. Therefore, geometric MR sets up a vicious cycle and has been associated with worsened survival even with mild MR and few overt symptoms of heart failure [26–28].
Geometric MR in the setting of Ischemic MR is slightly different. In this instance the remodeling of the LV is predominantly in the region of the inferobasal wall. This area is akinetic, dyskinetic or just lagging the normal systolic function of the left ventricle. As the mitral leaflets try to coapt in systole, the lagging inferobasal wall has an effect on the posterior leaflet of the mitral valve. The downward pull or restriction of the posteromedial posterior leaflet in the P2–P3 area prevents good apposition of the two leaflets. The leads to eccentric mitral regurgitation.
Compared to valvular MR, the natural disease progression of geometric MR is rapid and prognosis remains poor. Geometric MR is a prevalent complication of end-stage cardiomyopathy and may affect up to 60 % of all heart failure patients as a pre-terminal or terminal event [28–30]. Ischemic MR, that is, geometric MR in ischemic DCM doubles the mortality after myocardial infarction with a graded decrease in survival related to the severity of MR. Figure 9.4 reiterates the ischemic mechanism overlaid on the geometric components. It is reported that in ischemic DCM, the 5-year survival is 60 % without MR, 45–50 % with mild MR and 30–35 % with more than moderate MR [31, 32]. Decreased LV function reflected by a decreased ejection fraction further predicts a worse prognosis [33, 34]. The prognosis of ischemic DCM with MR is generally even worse than idiopathic DCM with MR [28].
However, the appropriate timing of intervention for chronic, geometric MR is still very controversial because of the higher operative risk and poorly defined late outcome measures.
With normal ventricular geometry, the redundant mitral leaflets are responsible for a zone of coaptation that is more than twice the area of the mitral valve orifice [10]. As the failing ventricle dilates, the multifactorial mechanisms of the progressive expansion of the mitral annulus and the dislocation of papillary muscles and LV wall leads to incomplete leaflet coaptation and a regurgitant jet of functional mitral insufficiency.
As more leaflet tissue is utilized for coverage of the enlarging orifice, a critical reduction in leaflet tissue available for coaptation is reached so that leaflet coaptation becomes ineffective and that a central regurgitant jet type of geometric MR develops [8, 35, 36]. In studies of patients with DCM, those with MR have significantly greater mitral leaflet orifice surface area and significantly larger dimensions of the mitral valve annulus than those without MR. However, these changes are minor compared to patients with fibro-elastic degeneration or myxomatous disease. Indeed, in many patients the annulus maybe normal in size. Chordal length and papillary muscle length are not significantly different in patients with cardiomyopathy, with or without MR [8]. It is also reported that pharmacologic reduction in the dynamic MR through the medical treatment of heart failure was through a reduction in the regurgitant orifice area which was related to the decreased mitral annular distention [36].
Therefore, the most significant determinant of leaflet coaptation in geometric MR is the diameter of the mitral valve annulus. This forms the basis of the approach to downsizing, and overcorrecting a complete MV ring annuloplasty. The spatial mis-alignment of the subvalvular mitral valve apparatus, that is papillary muscle – LV wall dislocation, also contributes to inability of leaflet coaptation [9, 37, 38]. As the ventricle dilates, the distance and the angle of the papillary muscles tends to become obtuse rather than acute, forcing the mitral valve leaflet coaptation zone apart. In addition, there is a large apical force that pulls the papillary muscle and chordal apparatus in an inferior and lateral direction and there is a weak closing force of the poorly functioning LV. All of these elements result in the loss of the zone of coaptation and subsequent regurgitation. This is illustrated in Fig. 9.5. The downsized and overcorrected complete ring annuloplasty also has some effect on the of the angle and the distance of de-arranged subvalvular mitral valve apparatus by indirectly providing more leaflet tissue for zone of coaptation and by directly influencing the reduced septal-lateral diameter at the level of papillary muscle through the continuum of papillary muscle-LV wall complex.
Although significant undersizing of a complete ring annuloplasty is performed to increase coaptation, no systolic anterior motion (SAM) of the anterior leaflet, or mitral stenosis was noted in the Bolling series. SAM is not usually seen in the setting of a large aorto-mitral angle and increased LV size, both conditions that are seen in DCM.
In contrast to primary or anatomic MR, geometric MR is also reported to have dilatation along the anterior aspect of the annulus [39]. This could possibly explain why the partial ring annuloplasty, rather than a complete ring annnuloplasty appears unlikely to produce a sustained long term result [40].
With ischemic DCM, geometric MR is furthermore compounded by the dynamic and regional changes of LV muscle function and geometry. Ischemic papillary muscle dysfunction which is traditionally defined as the cause of geometric MR is a misnomer. It is not an isolated disorder of the contractive function of the papillary muscle, which is often preserved. There is often disturbance in the coordinated geometry of mitral valve complex including the annulus, chordae tendinae, papillary muscles and the LV wall. It is reported that MR cannot be reproduced through direct damage causing fibrosis of papillary muscle and it may actually decrease with papillary muscle ischemia [41, 42]. This is why “papillary muscle – LV wall dislocation “rather than” ischemic papillary muscle dysfunction” has been more recently used to describe this condition [43].
Geometric Mitral Valve Repair (University of Michigan Experience)
In 1993, Dr. Bolling and his group began to use mitral valve repair techniques very cautiously to selected DCM patients with severe MR who were suffering from progressive severe heart failure and were not eligible for transplantation, on the assumption that mitral valve is the geometric functional component of the LV and that geometric MR, in which mitral valve apparatus itself is originally normal, is the functional and geometric problem of the LV [17].
In 1995, a small initial series of patients at the University of Michigan was reported describing the early outcome (1993–1994) of mitral valve reconstruction in 16 consecutive patients with DCM and severe MR, refractory to maximum medical therapy. In that study, 16 patients (11 men and 5 women) ranged in age 44–78 years (64 ± 8 years) underwent mitral valve reconstruction with a simple, undersized, flexible, complete ring annuloplasty. The ejection fraction was 9–25 % (16 ± 5 %). Two patients were listed for transplantation. No postoperative patients required support with an intra-aortic balloon pump. There were no operative or hospital deaths and mean hospital stay was 10 days. There were three intermediate term deaths at 2, 6 and 7 months after procedure, and the 1-year actuarial survival rate was 75 %. At a mean follow-up of 8 months, all remaining patients were in NYHA class 1 or 2, with a mean post-operative ejection fraction of 25 ± 10 % [17].
Historically, while significance of MR in CHF was recognized and attempts were made to treat this surgically with mitral valve replacement, early surgical correction was associated with poor outcomes and surgical teaching evolved to enforce this idea [44–47]. Consequently, these patients were not considered operative candidates due to the prohibitively high morbidity and mortality in this patient population [48–52]. The prevailing surgical thought was that MR provided a “pop-off” effect for these impaired ventricles to function and that by removing the MR and the “pop-off” effect the LV was compromised which led to the high operative mortality. However, the poor outcomes of mitral valve replacement in that era were probably from the adverse consequences of the excision and the disruption of the annular-chordal-papillary muscle continuity, which has significant importance on LV systolic function [11, 53–56]. It has been demonstrated in a number of studies that preservation of the annulus-papillary muscle continuity is of paramount importance to preserve LV function, and this is even more critical in patients with severely compromised LV function [12, 57–62]. Preservation of the mitral valve apparatus and LV in mitral valve repair has been demonstrated to enhance and maintain LV function and geometry with an associated decrease in wall stress [63]. This procedure in degenerative valvular MR has been shown to be safe, with a significant decrease in operative morbidity and mortality, and good long-term outcomes [64–69]. There is no “pop-off” effect seen because the total workload to produce the same forward flow without MR is proportionally decreased compared to the setting of volume overload with MR, even though the total resistance to the LV is decreased by MR [70, 71].
Feasibility of a surgical treatment of geometric MR with DCM had been established through the use of the simple, undersized, flexible complete ring annuloplasty. Pre- and post echocardiography data showed some changes in parameters related to hemodynamic and geometric improvement. The basal angulation of the heart was changed by undersizing, overcorrecting the annulus and the elliptical shape of the heart became re-established such that not only was MR acutely obliterated, but the cardiac geometry was also influenced, allowing the subsequent reverse remodeling [17–20]. Figure 9.6 shows schematically how this concept might work.
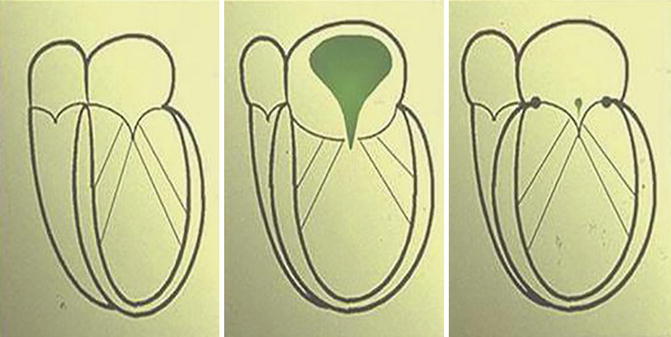

Fig. 9.6
Correction of coaptation by use of an undersized annuloplasty ring
At the University of Michigan (1993–2003), 215 patients with end-stage cardiomyopathy and refractory MR underwent mitral valve repair with a simple, undersized, complete ring annuloplasty. The range in age was 30–87 years (64 ± 12 years). Ejection fraction was 6–30 % (20.8 ± 6 %). Pre-operative NYHA class was 3.1 ± 0.9. A large number of patients (64/215, 30 %) had had a previous open-heart procedure. Thirty-day mortality was 4.7 % (10/215) for all mitral repairs. Post-operative low cardiac output syndrome was 2.3 % (5/215). Complication rates were low with CVA/TIA 2 % (4/215), prolonged ventilation 6 % (14/215), total infection 5 % (11/215), renal failure requiring dialysis 1 % (2/215) and reoperation for bleeding 0.5 % (1/215). Average ICU stay was 2.67 days and average hospital stay was 7.8 days. One and 2 year actuarial survival rates were 80 % and 70 %, respectively.
These techniques and principles have been adopted throughout the surgical community as the contribution of the mitral annulus, chordal structures and LV geometry to LV function were further understood [14, 72–77].
Numerous studies, from the other major surgical institutions, of mitral reconstruction in ischemia and idiopathic DCM, have also noted acceptable low operative mortality and been successful in relieving MR in CHF patients.
Chen from Brigham and Women’s Hospital published in 1998 a report of 81 patients undergoing mitral valve surgery for mitral valve regurgitation in dilated cardiomyopathy with 11 % total perioperative mortality. In this series LVEF improved from 24 % to 32 % and there was an improvement in NYHA class from 3.2 to 1.6. Estimated survival in this study was 73 %, 58 %, and 38 % at 1, 3, and 5 years respectively [78].
Bishay and colleagues in Cleveland Clinic Foundation reported in 2000 on 44 patients with isolated mitral valve surgery with LV mean ejection fraction (LVEF) below 35 % with a 2.3 % mortality. In this series LVEF improved from 28 % to 36 % and NYHA functional class decreased from 2.8 to 1.2. Survival was 89 %, 86 %, and 67 % at 1, 2, and 5 years respectively. Furthermore they noted a decrease in the left ventricular chamber sphericity [79].
Bitran in Israel reported in 2001 a decrease in heart failure symptoms and a decrease in New York Heart Association (NYHA) functional class without any operative mortality in 21 patients with LV mean ejection fraction (LVEF) below 25 % [80].
Rothenburger from Germany in 2002, described 31 patients with isolated mitral valve surgery with LV mean ejection fraction (LVEF) below 30 % with 6.5 % mortality. In this series LVEF improved from 23 % to 36 % and NYHA functional class decreased from 3.3 to 2.1. Survival was 91 %, 84 %, and 77 % at 1, 2, and 5 years respectively. Freedom from readmission for heart failure was 85 %, 79 % and 68 % at 1, 2, and 5 years respectively [81].
More recently Calafiore and associates in Italy published in 2004 a series of mitral repairs in 91 DCM patients (64 ischemic and 27 idiopathic). In this study, mitral valve annuloplasty was performed in 64 patients and 27 underwent a mitral valve replacement. The 30-day mortality rate was 4.4 %. LVEF improved from 27 % to 32 % and NYHA functional class improved from 3.5 to 2.1 in the 69 survivors. Interestingly, the probability of being alive at 5 years was 78 % and was higher in mitral valve repair group (81 %) than in mitral valve replacement group (67 %). The probability of being alive at 5 years with an improvement of at least one NYHA class was 66 % and was higher in the mitral valve repair group (77 %) than in mitral valve replacement group (52 %). Published series have come from numerous other units and countries and now constitute hundreds of cases performed with less than 5 % mortality [82].
The ACORN passive ventricular restraint device has also been studied in this group of patients. In the most recent ACORN series, a prospective, randomized and controlled, multi-institutional, multi-surgeon experience in 193 patients with MR and a mean EF of 23.9 %, LVEDD 69.7 mm, in which most of them underwent undersizing Geometric mitral valve repair, showed that mitral valve surgery in DCM patients with MR could be performed with a 1.6 % 30 day mortality. One-year and 2-year survival was 86.5 % and 85 % respectively. This Acorn trial is also a unique opportunity to assess the long-term efficacy of mitral valve surgery in patients with heart failure. In this report, surgery patients had a significant increase in 6 min walk times immediately after surgery and were associated with significant improvements in two different quality of life measures. Surgery patients were also associated with a remarkable reversal of LV remodeling, as manifested by a decrease in LVEDV and LVESV, an improvement in LVEF and sphericity index, and a reduction in LV mass. MR was effectively reduced and maintained for at least 18 months of follow-up [83]. However, the Acorn CSD has not been approved by the FDA and this is a trial of historic interest.
The latest set of data in mitral valve reconstruction in patients with heart failure have emerged from the NIH sponsored multi-center studies looking at these vexing issues.
One of these was a study involving 301 patients with moderate ischemic mitral regurgitation that were randomized to CABG alone or CABG plus mitral-valve repair (combined procedure). The primary end point was the left ventricular end-systolic volume index (LVESVI), a measure of left ventricular remodeling, at 1 year.
At 1 year, the mean LVESVI among surviving patients was 46.1 ± 22.4 ml per square meter of body-surface area in the CABG-alone group and 49.6 ± 31.5 ml per square meter in the combined-procedure group (mean change from baseline, −9.4 and −9.3 ml per square meter, respectively). The rate of death was 6.7 % in the combined-procedure group and 7.3 % in the CABG-alone group (hazard ratio with mitral-valve repair, 0.90; 95 % confidence interval, 0.38–2.12; P = 0.81). The rank-based assessment of LVESVI at 1 year (incorporating deaths) showed no significant between-group difference (z score, 0.50; P = 0.61). The addition of mitral-valve repair was associated with a longer bypass time (P < 0.001), a longer hospital stay after surgery (P = 0.002), and more neurologic events (P = 0.03). Moderate or severe mitral regurgitation was less common in the combined-procedure group than in the CABG-alone group (11.2 % vs. 31.0 %, P < 0.001). There were no significant between-group differences in major adverse cardiac or cerebrovascular events, deaths, readmissions, functional status, or quality of life at 1 year (ClinicalTrials.gov number, NCT00806988) [84].
An insightful editorial by Sundt into the findings of this article reported that “Entry into this study required multivessel coronary artery disease and a moderate degree of mitral regurgitation without structural valvular abnormalities; a previous myocardial infarction was not a requirement, and indeed only about 65 % of patients had such a history. The inclusion of patients with mitral regurgitation secondary to reversible ischemia may well explain why so many had an improvement in their mitral regurgitation with bypass alone. This may also explain in part why recurrent mitral regurgitation after repair was present in only about 10 % of patients, not the 30 % reported by others. It is possible that the authors set themselves up to show no significant difference between treatment groups” [85].
Many lessons have been learned in these geometric MR patients and further understanding and advances continue to be published in clinical and basic studies. The anterior trigone to trigone distance may enlarge variably in this type of CHF patient and it is not a good or useful guide for ring sizing. Anatomic and laboratory studies have confirmed this dilation of the anterior trigone to trigone distance from ischemia and dilated cardiomyopathy [39, 86, 87].
It has recently been reported that partial ring annuloplasty, not complete ring annnuloplasty is more likely to fail requiring repeat intervention [86]. Furthermore, undersizing or downsizing has been shown to be not only helpful in abolishing MR, but also in remodeling the base of the heart from the “bending” of the mitral annulus. This geometric re-arranging may have helped reestablish an ellipsoid shape to the base of the LV cavity. However, there is also emerging evidence that despite good early results, undersized annuloplasty does not serve all patients well in the short or intermediate term.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


