Mitral Valve Repair
Javier G. Castillo
David H. Adams
The mitral valve (MV) is a dynamic assembly of independent anatomical components including annulus, leaflets and commissures, chordae tendinae, papillary muscles, and left ventricle. During systole, a coordinated interaction of all these anatomic structures seals the valve against left ventricular pressure and allows one-way forward blood flow through the left ventricular outflow tract in practically frictionless fashion. Minimal anatomical alterations of the MV components may result in reduction of leaflet coaptation or opening and, consequently, mitral regurgitation (MR) or mitral stenosis (MS) respectively. While even a normal competent valve may allow a trivial amount of reversed flow, more than a trace of MR is considered pathologic. Mild to moderate MR might be tolerated indefinitely as long as it doesn’t worsen. However, more severe degrees of MR cause left ventricular remodeling, reduced forward cardiac output, neurohumoral activation, left ventricular damage, heart failure, and ultimately death.
The natural history of MR depends intimately on its etiology, the severity of left ventricular volume overload as well as its contractile performance, and the appearance of overlapping clinical conditions secondary to reversal flow such as atrial fibrillation or pulmonary hypertension. Degenerative disease is the most prevalent cause of surgical MR in the United States (representing 60% to 70% of cases). Other major causes of surgical MR are ischemic cardiomyopathy (20%), endocarditis (3% to 5%), and rheumatic disease (2% to 5%). Currently, MV repair is recommended in most circumstances as a primary surgical therapy versus valve replacement, particularly in asymptomatic patients and specific etiologies such as degenerative disease.
Currently, there is no strong surgical indication for patients with a severity of less than severe MR, except in symptomatic patients where there is a high suspicion of underestimation. In such patients, exercise testing is critical to clarify the decision-making. While there is the concern that moderate MR will likely worsen over time, the majority of patients should be treated with close observation until the MR has progressed in severity.
The standard class I indications for MV surgery are the onset of symptoms and/or left ventricular dysfunction. However, there is a significant trend toward offering early surgical intervention for asymptomatic patients who have preserved left ventricular function based on two tenets: (a) Equal or above 95% repair rate based on preoperative evaluation of the valve anatomy and lesions and (b) according to the literature patients with severe MR will develop a significant adverse event (symptom onset, atrial fibrillation, left
ventricular dysfunction) within 5 years of diagnosis. In addition, recent reports have suggested survival benefit with early surgery, providing additional support to the argument for earlier elective intervention. High-volume centers have shown a near 100% repair rate for degenerative MV disease, with an operative risk of less than 1%. Matching surgical expertise and experience to the complexity of a specific valve morphology and patient is important in the modern era to assure high repair rates.
ventricular dysfunction) within 5 years of diagnosis. In addition, recent reports have suggested survival benefit with early surgery, providing additional support to the argument for earlier elective intervention. High-volume centers have shown a near 100% repair rate for degenerative MV disease, with an operative risk of less than 1%. Matching surgical expertise and experience to the complexity of a specific valve morphology and patient is important in the modern era to assure high repair rates.
The presence of severe preoperative symptoms (New York Heart Association [NYHA] functional class III or IV) has been observed to confer a poor prognosis for patients postoperatively, even if left ventricular function is preserved. The onset of symptoms translates as a variation in the normal cardiac physiology as MR has begun to compromise cardiac output. In addition, there may be a small risk of sudden death in patients who have developed symptoms. It is extremely important to correct MR at the onset of even mild symptoms since watchful waiting for the progression of symptoms seems dangerous.
Left ventricular dysfunction secondary to MR may or may not induce symptoms. However, if not corrected, dysfunction may become permanent leading to a poor surgical outcome and eventually to death. In the absence of symptoms, two indicators of left ventricular dysfunction have been accepted: (a) An ejection fraction below 60% and (b) left ventricular end systolic dimension equal or greater than 40 mm. Classic mitral literature have demonstrated superior survival and ventricular remodeling in those patients who do not meet these criteria.
Decision-making in patients with severe secondary or functional MR (not requiring concomitant coronary artery bypass grafting [CABG] surgery) is more complex and challenging. In this setting, several factors need to be taken into account including the negative impact of ischemic MR on mid-term survival, the impact of a potential concomitant CABG on surgical outcomes, and, of course, the choice of valve repair versus replacement. In this regard, the clinical literature remains contradictory, making it difficult to synthesize and distill the information into concrete and practical clinical recommendations. In patients with NYHA functional class I or II, surgery is only indicated in the presence of pulmonary hypertension and an acceptable ventricular function (left ventricular ejection fraction [LVEF] >30% and left ventricular end-diastolic dimension [LVEDD] 41 to 64 mm); otherwise, the initial therapy is medical management. This may often result in reduction of severity of regurgitation, with a varying degree of symptom improvement. Cardiac resynchronization therapy with biventricular pacing should be employed in patients with severe secondary MR who show evidence of dyssynchrony. Alternatively, in patients with NYHA functional class III or IV, the initial therapy is MV surgery unless there is severe LV dysfunction (LVEF ≤30% and LVEDD ≥65 mm) or no viable myocardium. In this case, advanced heart failure therapies are indicated.
Preoperative workup for patients undergoing MV repair should include the acquisition of a comprehensive medical history with an emphasis on cardiovascular details, physical examination with additional dental clearance (a letter from a dental specialist is requested), routine laboratory tests (complete blood count, basic metabolic panel, coagulation profile, liver function tests, spirometry in patients with airway disease or chronic obstructive pulmonary disease [COPD], type and screen, methicillin-resistant staphylococcus aureus [MRSA] swabs, urine dipstick and hemoglobin A1C in diabetic patients), ECG (atrial fibrillation can be corrected with a concomitant maze procedure) and chest x-ray. In addition, all patients should have a timely preoperative consultation with anesthesia, particularly those undergoing surgery on the day of admission.
Coronary catheterization is indicated for most patients over 50 years of age or in younger patients with congenital malformations (right and left), ventricular dysfunction (right and left), or risk factors for coronary artery disease. A computed tomography angiogram is indicated in younger patients without any of the aforementioned conditions. In addition, a computed tomography of the chest without contrast will be obtained in the setting of reoperative sternotomy (assessment of the proximity of the
cardiac structures to the sternum) or in patients over 70 years old for assessment of aortic calcifications. A head and abdominal computed tomography are indicated in patients with endocarditis not undergoing emergent surgery in order to rule out the presence of embolic foci.
cardiac structures to the sternum) or in patients over 70 years old for assessment of aortic calcifications. A head and abdominal computed tomography are indicated in patients with endocarditis not undergoing emergent surgery in order to rule out the presence of embolic foci.
In general, beta-blockers, diuretics, and other medications for noncardiovascular conditions should be stopped the night before surgery. However, angiotensin-converting-enzyme (ACE) inhibitors should be discontinued at least 48 hours before the procedure to avoid intraoperative vasoplegia. Regarding anticoagulation, clopidogrel will be ideally discontinued at least 5 to 7 days before surgery, if possible (individualize patients with drug-eluting stents), aspirin 3 days before surgery, and coumadin 3 to 5 days before surgery (patients at a significant risk of thromboembolic complications are started on intravenous heparin or subcutaneous low molecular weight heparin). Patients with right ventricular dysfunction or elevated pulmonary hypertension (>60 mm Hg) may benefit from hemodynamic optimization with supplemental oxygen and intravenous nesiritide the day before surgery.
Positioning and Valve Exposure
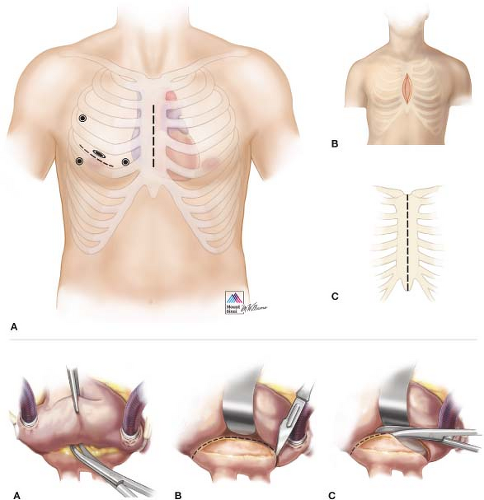
An excellent exposure of the MV is critical to the success of the surgical procedure. Multiple approaches have been classically described including median sternotomy (most common and standard approach in patients with complex lesions or in need of concomitant procedures), right anterolateral thoracotomy (almost abandoned in favor of minimally invasive approaches), and minimally invasive techniques. In turn, the latter can be subdivided into three different categories: Limited skin incision (with full or partial sternotomy), video assisted (through a right thoracotomy), or robot assisted (Fig. 16.1). Minimally invasive techniques other than median sternotomy through a limited skin incision will be reviewed and discussed in subsequent chapters.
Our routine institutional surgical access to the MV is median sternotomy through a limited small skin incision. The patient is placed in a supine position and all surgical landmarks are marked before skin prep (straight line from the sternal notch to the xyphoid and lower border of the ribs and breasts in female patients). A 7- to 8-cm incision is made in the lower portion of the chest and the incision is carried down to the sternum with cautery. The fascia overlying the pectoralis muscle must be freed to provide more laxity to the skin and the subcutaneous tissue for better retraction and exposure. Once the midline has been identified and marked from the xyphoid to the sternal notch, a reciprocating saw is used to divide the sternum from the xyphoid upwards as far as the skin incision allows. An Army-Navy retractor is subsequently placed in the divided portion of the sternum and turned 90 degrees to spread the two edges of bone and facilitate the division of the remaining sternum and manubrium with an oscillating saw. After hemostasis, a customized or a pediatric Cosgrove retractor (Kapp Surgical Instruments) is used to separate the sternum and allow access to the mediastinum.
After complete division of the pericardial sac in a standard fashion, the pericardial edge on the right side is sandwiched between the retractor blade and the right sternal edge to lift the right atrium and cava toward the skin edge, improving exposure. Subsequently, a clamp can be used to retract the aorta inferiorly into the field for cannulation. If the distance is suboptimal or the angle of exposure is too difficult, a Fem-Flex (Edwards Lifesciences LLC) cannula can be placed in the ascending aorta using a Seldinger technique. Cannulation of both cavas is then facilitated with the use of 24-Fr cannulas, and we typically use vacuum assistance. Finally, retrograde cardioplegia is used routinely on all mitral procedures via standard coronary sinus cannulation. The interatrial approach to the MV through Sondergaard’s groove remains the most efficient since it provides an excellent view and causes less tissue damage. The groove is usually
developed by carefully dissecting the interatrial space up to the fossa ovalis. After complete dissection, the roof of the left atrium should be fully exposed and cardiopulmonary bypass is instituted. The left atrium is then opened at the midpoint between the right superior pulmonary vein insertion and the groove. Exposure of the MV is achieved via a curvilinear incision extended longitudinally both superiorly to 1 cm from the superior vena cava and inferiorly to the midpoint between the right inferior pulmonary vein and the inferior vena cava. If further exposure of the left atrium is required, the pericardial reflection on both vena cavas is released and blunt dissection is used to free the lateral aspects of both veins for about 2 to 3 cm (Fig. 16.1).
developed by carefully dissecting the interatrial space up to the fossa ovalis. After complete dissection, the roof of the left atrium should be fully exposed and cardiopulmonary bypass is instituted. The left atrium is then opened at the midpoint between the right superior pulmonary vein insertion and the groove. Exposure of the MV is achieved via a curvilinear incision extended longitudinally both superiorly to 1 cm from the superior vena cava and inferiorly to the midpoint between the right inferior pulmonary vein and the inferior vena cava. If further exposure of the left atrium is required, the pericardial reflection on both vena cavas is released and blunt dissection is used to free the lateral aspects of both veins for about 2 to 3 cm (Fig. 16.1).
Valve Analysis
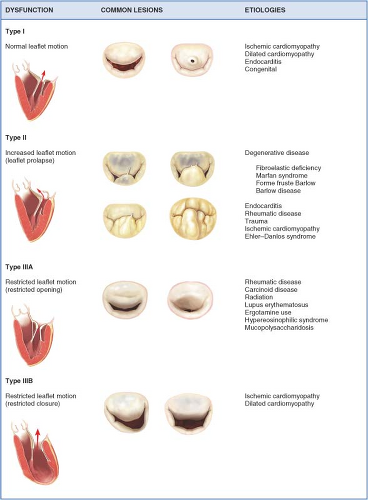
Meticulous echocardiographic and intraoperative interrogation (identification, localization, and assessment of magnitude) of the mitral lesions as well as determination of the mechanism of regurgitation (dysfunction) are essential to evaluate reparability. The differentiation of MV dysfunction (I, II, and III) as described by Carpentier is based on the position of the leaflet margins with respect to the normal mitral coaptation plane. Type I dysfunction implies normal leaflet motion, and the most common cause of significant MR is the perforation of one of the leaflets (e.g., endocarditis) or severe annular dilatation with a central regurgitant jet (e.g., long-standing atrial fibrillation). Type II dysfunction denotes excess leaflet motion generally secondary
to chordal elongation or rupture or myxomatous degeneration of the leaflets. Type III dysfunction involves restricted leaflet motion and typically results from retraction of the subvalvular apparatus (IIIA) or papillary muscle displacement secondary to leaflet tethering from LV remodeling or dilatation (IIIB) (Fig. 16.2).
to chordal elongation or rupture or myxomatous degeneration of the leaflets. Type III dysfunction involves restricted leaflet motion and typically results from retraction of the subvalvular apparatus (IIIA) or papillary muscle displacement secondary to leaflet tethering from LV remodeling or dilatation (IIIB) (Fig. 16.2).
Intraoperative valve inspection should be performed in a very systematic fashion (annulus, leaflets, chordae, and papillary muscles). The left atrial endocardial surface should be carefully examined for jet lesions, thrombi, and areas of calcification beforehand. The mitral annulus is then evaluated to assess shape, symmetry, any degree of dilation, as well as areas of severe calcification (potential impediments to place annular sutures). The leaflets are subsequently examined with a nerve hook to identify pathologic
segments (A1 to 3; P1 to 3, anterior and posterior commissures). After structural evaluation, the saline test helps to analyze “functional” leaflet lesions. Chordae tendinae are commonly interrogated for elongation, rupture, or more complex lesions such as chordal thickening, fibrosis, or fusion. Finally, the papillary muscles are examined for calcification, fusion, and/or abnormal ventricular insertion.
segments (A1 to 3; P1 to 3, anterior and posterior commissures). After structural evaluation, the saline test helps to analyze “functional” leaflet lesions. Chordae tendinae are commonly interrogated for elongation, rupture, or more complex lesions such as chordal thickening, fibrosis, or fusion. Finally, the papillary muscles are examined for calcification, fusion, and/or abnormal ventricular insertion.
Surgical Technique in Type I Dysfunction
The most common lesions in patients with type I dysfunction are symmetric annular dilation (most frequent scenario especially in patients with long-standing atrial fibrillation) or leaflet perforation (also known as windsock leaflet deformity) secondary to acute bacterial endocarditis (Fig. 16.3).
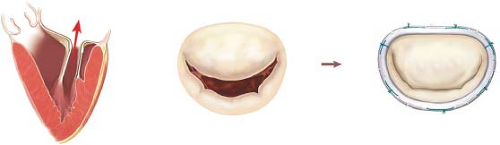
Every patient undergoing MV repair requires a remodeling annuloplasty in order to restore the native annular size and shape allowing full leaflet motion at the same time. This prevents any risk of recurrence by stabilizing the annulus (particularly the posterior aspect) with a prosthetic device (complete ring, open ring, or posterior band). Although remodeling annuloplasty is considered a very routine part of the repair, several anatomical structures are susceptible to injury during placement of annuloplasty sutures. Among them, in decreasing order of importance, the left circumflex coronary artery, the aortomitral curtain (including the left and the noncoronary aortic sinus with their corresponding aortic leaflets), and the coronary sinus (Fig. 16.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree