Mitral Valve Disease
Brian R. Lindman
Suzanne V. Arnold
High-Yield Concepts
Discrepancy between the mitral valve area (MVA) and mean gradient in MS may be related to cardiac output or heart rate
Wilkins score: Carefully review leaflet mobility, thickening, and calcification and subvalvular thickening to predict efficacy of balloon valvuloplasty
Eccentric regurgitant jet: Be suspicious that it might be severe
Grading regurgitation severity: Do not rely on one measurement alone
Key Views
Parasternal long axis—initial screening for mitral stenosis (MS) and mitral regurgitation (MR) severity; evaluation of subvalvular apparatus
Parasternal short axis—planimetry of MVA
Apical views—quantitative assessment MS and MR: Mean gradient, proximal isovelocity surface area (PISA) and volumetric assessments, LA and LV size and function
TEE—higher resolution views for MR etiology and severity (e.g., PISA, pulmonary vein reversal) and for Wilkins score; important for planning interventions
Severity of Mitral Stenosis
See Table 10-1.
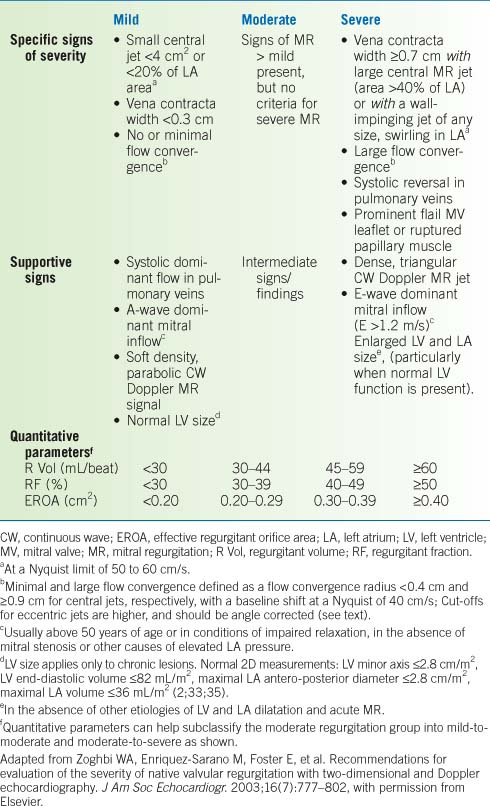
Table 10-1 Criteria for Determining Severity of Mitral Valve Stenosis | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Severity of Mitral Regurgitation
See Table 10-2.
Table 10-2 Criteria for Determining Severity of Mitral Valve Regurgitation | |
|---|---|
|
Anatomy
Leaflets
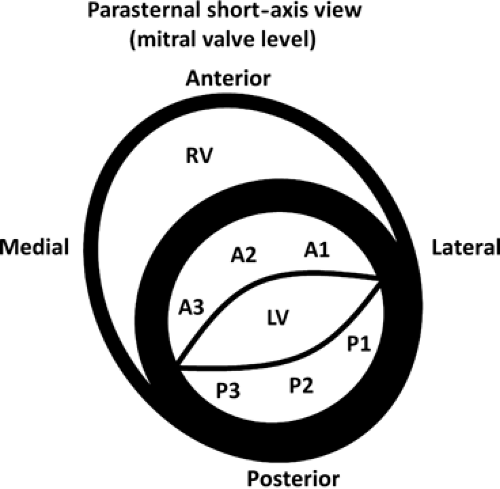
Anterior and posterior leaflets each have three scallops (Fig. 10-1).
Annulus
The junction between atrium and ventricle and the place where the mitral leaflets insert.
There is an anterior and posterior portion of the annulus corresponding to the respective leaflets. The anterior portion is attached to the right and left fibrous trigones and is structurally more supported.
The annulus may dilate leading to functional regurgitation or calcify leading to stenosis.
Subvalvular Apparatus
Papillary muscles: Anterior–lateral and posterior–medial; the anterior–lateral papillary muscle usually receives dual blood supply (LAD and LCX), whereas the posterior–medial papillary muscle usually has single blood supply (either LCX or RCA).
Chordae tendinae: Primary, secondary, and tertiary chords connect the papillary muscles to both valve leaflets; these can elongate, shorten, rupture, calcify, or fuse.
Ventricle
The ventricular size and shape impact the function of the mitral valve. As the ventricle dilates and becomes more spherical, the papillary muscles become apically displaced and can restrict the closure of the mitral leaflets, leading to MR.
Left Atrium
If the left atrium dilates, this can lead to annular dilation and affect the closure of the mitral leaflets, leading to MR.
Key Point:
Unlike the tricuspid and pulmonic valve, which is separated by the RV infundibulum the mitral and aortic valve, are in direct continuity, separated only by a fibrous connection. This has ramifications for pathology such as endocarditis, which can therefore spread between both valves and cause annular and aortic root abscess.
Mitral Stenosis
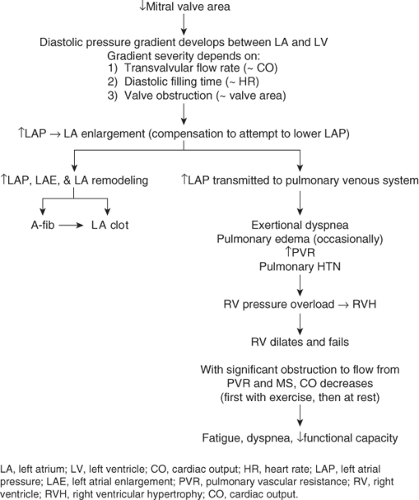
Pathophysiology
See Figure 10-2.
Etiology
Rheumatic (most common)
- 2/3 are female
- May be associated with MR
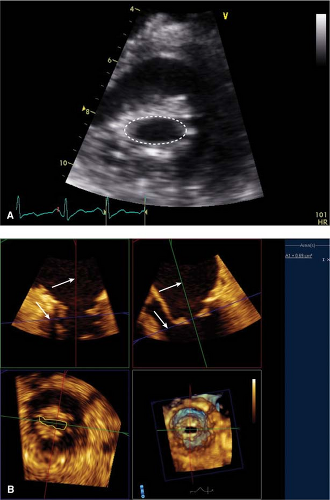
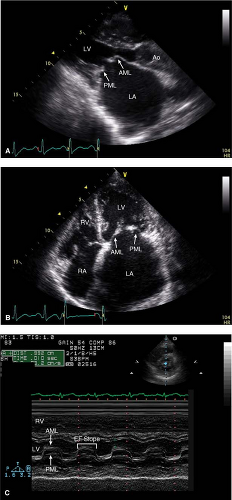
- Stenotic orifice often shaped like a “fish mouth” (PSAX) (Fig. 10-3) with doming of anterior leaflet (PLAX and apical views) (Fig. 10-4) and marked reduction in posterior leaflet motion
- Rheumatic fever can cause fibrosis, thickening, and calcification leading to fusion of the commissures, cusps, and/or chordae.
- As opposed to calcific MS this process starts in the subvalvular apparatus and extends to the leaflets with increasingly severe disease.
Other causes (less common)
- Congenital
- Mitral annular calcification (i.e., calcific MS)—process starts from annulus and extends to the leaflets. Significant calcification is required to impact the larger area of the mitral annulus compared to rheumatic MS which affects the leaflet tips
- Chest radiation
- MV prosthesis dysfunction
- Mucopolysaccharidoses
- Malignant carcinoid
- Systemic lupus erythematosus (SLE)
- Rheumatoid arthritis
- Iatrogenic due to surgery for MR: Oversewn mitral annuloplasty ring, MV clip/Alfieri stitch
- “Functional MS” due to restriction of left atrial outflow (MV leaflets are normal):
- Tumor (typically atrial myxoma)
- LA thrombus
- Endocarditis with large vegetation
- Cor triatriatum (congenital LA membrane)
- Tumor (typically atrial myxoma)
- 2/3 are female
Echocardiographic assessment of MS
2D assessment
Leaflets
Motion/mobility of the valve
Thickening
Calcification
Subvalvular apparatus
Chordal fusion, shortening, fibrosis, and calcification
Mitral valve area planimetry
The mitral valve orifice is traced in the parasternal short-axis view usually during mid-diastole.
This can be an accurate way to assess the severity of MS that is independent of flow, chamber compliance, and other valve lesions; however, it is also prone to error. While in the parasternal short-axis view, scanning should be done from apex to base to ensure that planimetry is being done at the leaflet tips. Sometime, reviewing the anatomy from the parasternal long-axis view can help identify the right plane in the short-axis view. This is a measurement where 3D imaging is particularly helpful in enhancing accuracy (Fig. 10-3).
Left atrial dimension
Significant MS can lead to substantial dilation of the LA, predisposing the patient to atrial arrhythmias and thrombus formation.
Wilkins score is graded as detailed in Table 10-3.
Doppler
MVA
Pressure half-time (PHT) method
PHT is the time it takes for the pressure across the mitral valve to decrease by one-half its original maximal value; it is measured by tracing the deceleration slope of the E wave on the CW Doppler profile through the mitral valve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree