Fig. 55.1
Different etiologies of mitral regurgitation. Schematic illustration of a normal mitral valve closure, degenerative MR with mitral valve prolapse, degenerative MR caused by flail leaflet, and functional MR (from left to right). Note the central MR in functional MR and pronounced excentric jet caused by the flail leaflet (Images courtesy of Abbott Vascular)
55.2.1 Primary MR
In primary (also termed organic or degenerative) MR, components of the valvular apparatus are directly affected. These can span from isolated destructive alterations of single components such as leaflet prolapse, leaflet perforation, and chordal or papillary muscle (PM) rupture (e.g., mitral valve prolapse in connective tissue abnormalities, endocarditis, ischemia, rheumatic disease) or other rare congenital causes to the co-occurrence of several of these pathologies. Primary MR is the most common cause for MR in the industrialized world [7, 9, 11]. It is described to develop and progress in stages. Initially, a condition with a risk of MR, such as a mild mitral valve prolapse (MVP) with normal coaptation or mild leaflet thickening and leaflet restriction represents a predisposing state for primary MR (stage A, Fig. 55.1). This state can decline into progressive MR (stage B, e.g., severe MP, rheumatic disease, or prior infective endocarditis (IE)) and asymptomatic severe MR (state C, e.g., loss of coaptation or flail leaflet), and finally, into stage with symptomatic severe MR (stage D, Fig. 55.1) [12]. As most patients with primary MR present with a normal left ventricular function and an otherwise unaffected heart, correction of primary MR in these patients is considered to be curative [12]. However, in some cases, primary MR can be encountered in CHF patients, who develop left heart dysfunction as a result of long-standing volume overload because timely treatment of primary MR has been missed. In other patients, mixed primary and secondary MR can be encountered. These patients initially exhibit mild primary MR and develop left ventricular dysfunction due to another underlying disease, such as myocardial ischemia, which then aggravates the already present primary MR.
55.2.2 Secondary MR
In heart failure patients, secondary MR (SMR) is more prevalent. In SMR, the structural integrity of the components of the valvular apparatus per se is preserved. Several mechanisms can contribute to the presence of SMR. One of the most commonly encountered mechanisms is MV leaflet tethering arising from displacement of the papillary muscles, either occurring as a consequence of regional wall motion abnormalities, particularly after an inferior or posterior myocardial ischemic event or as consequence of global LV dilatation and dysfunction (e.g., dilative cardiomyopathy or adverse remodeling after a large anterior myocardial infarction, Fig. 55.2). Further mechanisms include reduced closing forces due to LV dysfunction and functional (reduced contraction) and geometrical (flattening, dilation) changes of the MV annulus, which are in part only incompletely understood [1, 13].
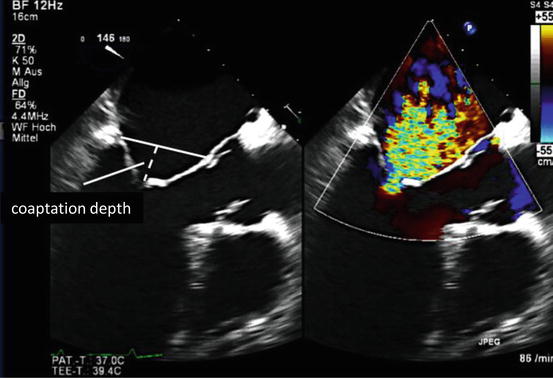

Fig. 55.2
Secondary mitral regurgitation. Transesophageal long axis view showing the mechanism of secondary mitral regurgitation with LV dilation, tethering of both leaflets, and increased coaptation depth. MR results due to decreased or absent coaptation (Image courtesy of Abbott Vascular)
Based on the underlying etiologies of heart failure, functional MR can be divided further into two subgroups, ischemic and nonischemic MR. Ischemic mitral regurgitation (IMR) is defined as functional MR caused by ischemic wall motion abnormalities or ischemic LV remodeling. Chronic IMR occurs after the healing phase of MI with an estimated prevalence of 20–50 % [3, 14]. The presence of IMR, even if it is clinically insignificant, represents an independent prognostic factor for worse outcome with a stepwise decline of survival as grade of IMR increases [3].
As described for primary MR, secondary MR also progresses in stages (stage A–D). In patients with CAD or cardiomyopathy, the valvular apparatus is initially not relevantly affected, therefore posing a risk for secondary MR (stage A). As LV geometry changes, regional wall motion abnormalities with mild leaflet tethering or annular dilation with decreased central coaptation induce progressive MR (stage B). This proceeds to severe but asymptomatic MR (stage C) and can ultimately result in severe tethering and relevant annular plane dilation with loss of central coaptation (stage D) [12]. As secondary MR is caused by left ventricular disease and by itself aggravates left ventricular disease due to the imposed volume overload, it is part of a self-perpetuating process, which accelerates functional and clinical deterioration.
Due to the complexity and dynamic nature of its underlying mechanisms, determination of MR severity by echocardiography requires some special considerations [12]. Thus, in contrast to primary MR, where the regurgitant jet has a circular cross-sectional area, the cross-sectional area of secondary MR typically is crescent shaped, which has important implications when assessing regurgitation severity with two-dimensional imaging techniques. Current guidelines therefore propose evaluation of the regurgitant jet in more than one plane, and three-dimensional techniques are rapidly evolving [15]. Additionally, dynamic changes due to exercise and loading conditions have a significant impact on severity of SMR. An exercise-induced increase of afterload has been proposed to increase displacement of papillary muscles away from the annular plane, thereby worsening MR by increasing tethering forces [16]. Grade of MR greatly depends on pre- and afterload conditions, explaining the effectiveness of diuretic and afterload-decreasing medication [17]. Likewise, volume status, systemic blood pressure, or anesthesia decisively effect severity of MR and might lead to significant misjudgment. In cases of uncertainty, exercise-stress echocardiography is warranted when a relevant MR is suspected [18]. The complex interplay between LV geometry and function, the mitral valve apparatus, and ultimately, the hemodynamics of the systemic circulation contributing to SMR make therapeutic decision-making a difficult task and explains why treatment strategies are not as clear-cut as for other valvular disorders [12].
55.3 Therapeutic Options
55.3.1 Medical Therapy
In primary MR or in the case of a relevant degenerative component of MR, medical therapy will only be of limited efficiency. Thus, ACE inhibitors (Chap. 36) and beta-blockers (Chaps. 5 and 8) can be used with the aim to reduce adverse LV remodeling in patients without an indication for valve intervention, although convincing evidence for slowing of MR progression or improved outcomes does not exist [19]. Administration of diuretics (Chap. 38) or vasodilators (Chap. 40), although potentially very effective, particularly in the symptomatic treatment of acutely decompensated patients, should not unduly delay valve repair or replacement once indicated. In contrast, the potential of medical therapy to positively influence secondary MR is better established [20–23]. On the one hand, it is based on unloading of the LV mainly by diuretics. On the other hand, more sustainable effects can be achieved by drugs targeting adverse LV remodeling, as they have the potential to interrupt the above described vicious circle between secondary MR and left ventricular dysfunction. Human and animal studies provide evidence that ACE inhibitors and beta-adrenergic blockade are capable of reducing MR by reverse remodeling and improving LV function [24–26]. Accordingly, the current AHA guidelines of valvular heart disease recommend optimized medical treatment in patients with reduced LV function and chronic secondary MR (stages B–D) including ACE inhibitors, ARBs, beta-blockers, and/or aldosterone antagonists (Class I, level of evidence: A) as an inalienable basis of heart failure therapy in patients with secondary MR and LV dysfunction (Table 55.1) [12]. In patients with ongoing symptoms and severe MR cardiac resynchronization, therapy is an option if general criteria are met (Table 55.1), and a surgical or interventional approach to correct valve function should be strongly considered (also see Chap. 54) [12].
Table 55.1
Recommendations for medical treatment in chronic severe secondary MR
1. Patients with chronic secondary MR (stages B–D) and HF with reduced LVEF should receive standard GDMT therapy for HF, including ACE inhibitors, ARBs, beta-blockers, and/or aldosterone antagonists as indicated (level of evidence: A) |
2. Cardiac resynchronization therapy with biventricular pacing is recommended for symptomatic patients with chronic severe secondary MR (stages B–D) who meet the indications for device therapy (level of evidence: A) |
55.3.2 Cardiac Resynchronization Therapy (CRT)
It is generally recognized that CRT can reduce severity of SMR in heart failure patients by restoring a more physiological timing of processes involved in mitral valve closure. Acutely, CRT was able to significantly reduce MR by optimizing timing of papillary muscle contraction in patients with left bundle branch block and dyssynchrony [27]. Moreover, CRT was shown to positively affect LV shape and functional parameters and reduce apical leaflet tethering, thereby significantly reducing MR [28, 29]. According to a small study involving 98 patients with severe secondary MR, 49 % of patients showed a reduction in MR grade that was associated with an improvement in survival compared to patients without a reduction in MR grade [30, 31]. According to current guidelines, CRT is indicated for symptomatic patients with chronic severe functional MR, if general criteria for resynchronization therapy are met (Class I, level of evidence: A) [12, 30, 32].
55.3.3 Surgery
In the absence of a high perioperative risk, reconstructive mitral valve surgery clearly is the gold standard of therapy in primary MR. In contrast, for secondary MR, the benefit of surgery is less well established as no prospective studies comparing surgery to conservative heart failure therapy exist, and observational data did not show a benefit for MV surgery over medical therapy in regard to survival [10], despite reverse LV remodeling and functional improvement [33]. However, there is an agreement that MV surgery is recommended for severe secondary MR when aortocoronary bypass surgery is indicated and that it should be considered when there is an option for revascularization [9]. The conventional approach for reconstructive surgery is restrictive mitral annuloplasty to decrease the anterior-posterior dimension of the MV annulus [34]. However, further techniques such as chord transection and methods for PM repositioning are critically debated. The complex nature of functional MR becomes evident by the fact that recurrence rates of relevant MR are around 30 % after MV reconstruction even in contemporary reports [34, 35]. One important aspect for recurrence of MR is believed to be a progression of ventricular disease particularly in patients with already severely dilated left ventricles [36]. In the future, scientific efforts are necessary to understand the influence of ongoing LV dysfunction and remodeling in order to be able to eliminate the factors causing high rate of recurrent MR.
Current guidelines recommend MV surgery in symptomatic patients with severe secondary MR (stages C and D) and who are undergoing coronary revascularization or aortic valve replacement (Class IIa, level of evidence C), patients who are considered severely symptomatic (NYHA III–IV) due to chronic MR (stage D, Class IIb, level of evidence B), or in patients with chronic moderate MR (stage B) who are undergoing cardiac surgery for other reasons (Class IIb, level of evidence C). In general, MV repair is recommended over MV replacement [12], although this concept has been challenged by a recent randomized trial failing to demonstrate a difference between both treatment with regard to LV remodeling or survival.
55.3.4 Interventional Approaches
An increasing number of interventional, catheter-based treatment options have been developed and are being tested in humans. Interventional approaches are designed primarily to reduce annular ring dilation or directly act on the leaflets, thereby increasing coaptation area.
55.3.4.1 Percutaneous Edge-to-Edge Repair with the MitraClip System
The MitraClip is a percutaneous transvenous procedure that aims to create an edge-to-edge repair of the MV resulting in a double orifice mitral valve mimicking the Alfieri surgical technique [37]. The procedure is performed under general anesthesia in the cath lab with a transvenous access usually via the right femoral vein. After transseptal puncture in the area of the fossa ovalis, a steerable guiding sheath (size 24 French) is placed in the left atrium. The inner lumen allows advancing the steerable clip delivery system toward the MV. After choosing an appropriate position (in most cases, the area of the greatest coaptation defect), the clip arms are opened and adjusted perpendicularly to the edge of the leaflets. Guiding of the procedure is performed mainly by transesophageal echocardiography (TEE, 2- and 3-dimensional) and by fluoroscopy. The clip is advanced from the LA, through the MV orifice to the LV side. Care has to be taken to avoid entangling in the chordae. After adjusting for the right position and angulation, the anterior and posterior leaflets of the mitral valve are grasped between the “gripper arms” and the “clip arms” (Fig. 55.3). By closing the clip, the leaflets are firmly gripped, forming a double orifice with approximated leaflet edges. Depending on the resulting MR reduction, the clip can be repositioned as long as the clip is attached to the delivery catheter. Once a satisfying result is obtained, the clip is released from the delivery system.
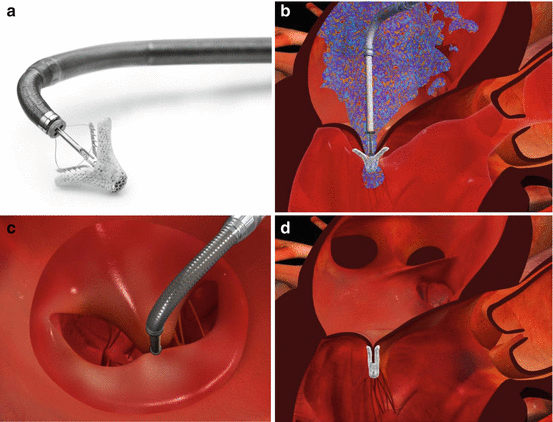

Fig. 55.3
MitraClip device and procedure. (a) Photograph of the MitraClip device attached to its delivery system. Leaflets are grasped between the clip arms and gripper arms. (b) Schematic illustrating leaflet grasping at the site of maximal mitral regurgitation. (c) Created double orifice mitral valve after grasping viewed from left atrium before final clip deployment. (d) Schematic of left ventricular outflow tract view after clip deployment (Images courtesy of Abbott Vascular)
In the only available RCT to date, the EVEREST II trial, 279 patients with moderate to severe or severe MR who were good candidates for MV surgery were randomized in a 2-to-1 fashion to percutaneous therapy or surgery. This study showed superiority for surgery, as freedom of the primary efficacy end point (death, surgery for mitral-valve dysfunction, or MR grade 3+ to 4+) at 12 months was significantly lower in the interventional than in the surgical group (55 % vs. 73 %, p = 0.007) [38], which was driven by a high rate of surgery for MV dysfunction in the interventional group. Reduction in MR and LV end-diastolic volume was higher in the surgical group than in the interventional group; however, surgical patients also exhibited a strong reduction in LVEF. In contrast, reduction in NYHA functional class was high in patients treated with the MitraClip [38]. After 4 years of follow-up, the difference with regard to the primary end point was no longer significant, and survival was identical in both groups [39]. It is of relevance to note that this trial predominantly included patients with primary MR (73 % of patients), whereas in the “real world,” more than two thirds of patients treated with the MitraClip have secondary MR. Importantly, a post hoc analysis of the EVEREST II trial revealed that effectiveness of the percutaneous approach was not inferior to surgery in patients with impaired left ventricular function and those with functional MR [38]. Moreover, a series of observational studies shows that MitraClip therapy is feasible and safe, even in high-risk patients with severely impaired LV function and that it is associated with symptomatic improvement [40–43]. Prospective, randomized trials comparing the MitraClip to optimized medical therapy for SMR are currently ongoing in Europe and the USA, and studies comparing the MitraClip with MV surgery are planned.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


