Mitral and Tricuspid Valve Disease
Mitral valve disease is a common valvular abnormality, resulting from various etiologies and having well-understood, varied, and interesting clinical manifestations. Tricuspid valve disease is less common, occurring most often as a functional result of left-sided heart disease and/or pulmonary hypertension.
MITRAL VALVE ANATOMY
The mitral valve apparatus consists of anterior and posterior leaflets, chordae tendineae, anterolateral and posteromedial papillary muscles, and mitral annulus. To be inclusive, it also includes the atrial and ventricular myocardium. Mitral valve dysfunction may result from aberrations of any portion of the mitral valve apparatus, as a result of mechanical, traumatic, infectious, degenerative, congenital, or metabolic causes.
MITRAL VALVE PROLAPSE
Mitral valve prolapse (MVP) is found in approximately 2% of the population and is equally common in men and women. It is the most common cause of mitral regurgitation (MR) in the United States. Most such patients have a minor amount of MR and therefore a benign prognosis, with no significant cardiovascular symptoms or manifestations such as congestive heart failure. The diagnosis of MVP is made usually by bedside physical examination, finding a mid-to-late systolic click or multiple clicks, sometimes associated with a late systolic or pansystolic murmur. The murmur becomes earlier and louder with standing and the Valsalva maneuver, resulting from reduction in preload, which brings the mitral leaflets closer together before left ventricular (LV) contraction. The murmur of mitral prolapse becomes softer and later with squatting due to an increase in preload.
The diagnosis of MVP is best confirmed echocardio-graphically. The best two-dimensional (2-D) echocardiogram criterion is leaflet displacement beyond the line of the mitral annulus in the long-axis view. Because of the saddleshaped configuration of the mitral valve, caution must be taken when MVP is diagnosed only from parasternal long-axis, apical four-chamber, and apical two-chamber views. M-mode criteria require 2 or 3 mm of displacement, either as late systolic or holosystolic hammocking (Fig. 34.1). The presence of an eccentric jet direction of MR makes the diagnosis of MVP more likely. In general, prolapse with leaflet thickness >5 mm is considered “classic” MVP, whereas prolapse with thinner valve leaflets is considered “nonclassic prolapse.”
Accepted indications for performing echocardiographic study in mitral prolapse include establishing the diagnosis, determining the severity of MR, evaluating leaflet morphology, and defining LV size and function.1 The list implies that echocardiography should be used when it can add information to findings available from history and the physical examination. Indications for echocardiography may also include exclusion of MVP in patients diagnosed with MVP when there is no clinical evidence to support the diagnosis (Table 34.1). Subsequent or serial echocardiograms are not usually necessary if the patient is asymptomatic, unless there are clinical indications of severe or worsening MR.
TABLE
34.1 Evaluation and Management of the Asymptomatic Patient in MVP

From Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–e148, with permission from Elsevier.
Most patients with mitral prolapse, even if there is a murmur, or if the echocardiogram shows significant MR, do not need antibiotic prophylaxis for endocarditis. Although that has been common practice prior to new guidelines that were published several years ago, the newest guidelines do not advise prophylaxis for native valve disease. Prophylaxis during procedures likely to cause bacteremia (including teeth cleaning) is recommended if the patient has had previous endocarditis or if a prosthetic valve has been implanted (Table 34.2).
TABLE
34.2 Recommendations for Infective Endocarditis Prophylaxis in Valvular Heart Disease
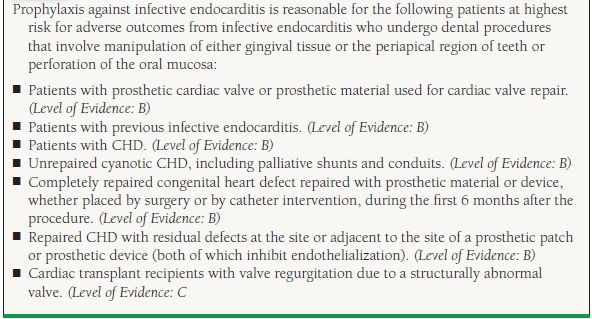
Adapted from Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease). J Am Coll Cardiol. 2008;52:e1-e142; Wilson W Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736-1754. Ref. (5,6).
The natural history of MVP is frequently benign. Follow-up studies in large population samples show that most patients with MVP do quite well, and most do not develop any significant congestive heart failure, atrial fibrillation (AF), stroke, or syncope. After a prolonged asymptomatic interval, a small percentage of patients with MVP develop more severe MR, ruptured mitral valve chordae (flail), left atrial and ventricular enlargement, or AF.2 In addition, with gradual progression of MR, LV dilatation and dysfunction may occur, leading to congestive heart failure. A substantial negative effect on survival has been seen in patients who develop LV dysfunction, AF, left atrial enlargement, age >50 years, and flail mitral leaflet. Recently, quantitatively severe regurgitation has also been associated with adverse prognosis.3,4
The development of infective endocarditis in patients with MVP is another mechanism of increase in MR. Predictors of infective endocarditis in patients with MVP include male gender, age >45 years, the presence of MR, and leaflet thickening and redundancy Patients with MVP with significant MR also have a small but significantly increased risk of sudden death, most likely secondary to ventricular tachyarrhythmias, which may be predicted by concomitant systolic dysfunction.
MVP has been associated with a pattern of multiple nonspecific symptoms such as palpitations, atypical chest pain, syncope, and anxiety, and this constellation has been frequently termed the “mitral valve prolapse syndrome.” No such associations have been found in multiple studies, but a small group of patients may have a complex set of symptoms associated with MVP For example, a few studies have shown a pattern of autonomic dysfunction, with increased catecholamines and decreased vagal tone, in patients with MVP.
The mainstay of medical management of patients with MVP is reassurance. Beta-blockers are the treatment of choice for patients with increased adrenergic symptoms such as palpitations, chest pain, or anxiety, though some of these effects may be the placebo effect. In patients with MVP and transient ischemic attacks (TIAs) or stroke, the treatment is usually just aspirin (81 to 325 mg/d). Warfarin may be indicated in some patients with MVP and recurrent TIA or stroke. In addition, in patients with AF, there should be a low threshold for instituting anticoagulation, individualized for the patient’s risks of stroke versus bleeding. Surgery for MVP is only a consideration in patients with more severe MR, similar to other forms of nonischemic MR, which is discussed later.
ACUTE MITRAL REGURGITATION
Acute MR is an uncommon medical condition of grave importance, requiring urgent medical and often surgical intervention. Acute MR also occurs due to disruption of mitral valve leaflets, chordae tendineae, or papillary muscles that may result from infective endocarditis, acute myocardial infarction, trauma, or rheumatic fever. The most common cause is probably acute myocardial ischemia leading to acute LV enlargement, severe MR, and acute pulmonary edema.
High left atrial pressure and reduced left atrial compliance secondary to severe MR are the mechanisms of pulmonary edema. A less common complication of severe acute MR is reduced forward flow with cardiogenic shock. Acute MR usually presents as sudden and marked increase in congestive heart failure symptoms, with weakness, fatigue, dyspnea, and sometimes respiratory failure and shock. Peripheral vasoconstriction, pallor, and diaphoresis are usually associated presenting signs. In some patients, a loud systolic murmur and a diastolic rumble or third heart sound are heard. In others, a very soft murmur or no murmur is heard, because the severity of MR and the lack of atrial compliance lead to midsystolic equalization of pressures between the left atrium and ventricle midway through systole. In addition, the acute nature of the condition obscures the mitral murmur by other aspects of the patient’s distress, including orthopnea, precluding a good exam in the left lateral decubitus position.
Echocardiography is the diagnostic procedure of choice. In acute coronary syndromes, emergency catheterization and cardiac surgery are life saving. There is little need for contrast LV angiography, except in cases where there is discrepancy in clinical and noninvasive findings. In some cases, hemodynamic measurements and monitoring may also be helpful in management.
Acute MR after myocardial infarction is discussed in detail in another chapter of this book. It is the cause of about 7% of cases of cardiogenic shock after myocardial infarction. The onset of the MR is most commonly between days 2 and 7 after myocardial infarction. The MR in most patients, like our example at the beginning of this chapter, involve a functional mechanism, from apical tethering of normal leaflets as a consequence of acute LV dysfunction and enlargement. Focal infarction most commonly involves the posteromedial papillary muscle, because it derives its blood supply solely from one artery, the right coronary artery. In contrast, the anterolateral papillary muscle has a dual blood supply, often derived partly from the circumflex and partly from left anterior descending artery. Despite the devastating effects of acute severe MR, the infarct size is not always large, with some smaller infarctions (<25% of LV), with a mild to moderate enzyme leak.
Hemodynamic stabilization with prompt surgical intervention is the most effective therapy for most cases of acute MR. Vasodilator therapy with intravenous nitroprusside and nitroglycerin may lead to decreased MR, increased forward flow, and reduced pulmonary congestion. Intra-aortic balloon pump (IABP) counterpulsation is also effective in reducing regurgitant volume and LV filling pressure, while increasing forward output and mean arterial pressure, and is frequently used for initial stabilization, as a bridge to prompt surgical intervention. However, if appreciable aortic regurgitation (AR) is present, IABP is contraindicated because it worsens the AR.
CHRONIC MITRAL REGURGITATION
The physiology of MR includes a number of classical features (Fig. 34.2). The patient with MR of any cause usually has a pansystolic murmur, best audible at the apex, often radiating to the axilla. In severe MR, this murmur also may be heard in the left paravertebral area of the back. The pulmonic component of the second heart sound may be louder than normal if there is pulmonary hypertension. An inflow sound (a third heart sound or an early diastolic rumble) at the apex may be heard in some patients if the MR is severe. The apical impulse is enlarged, displaced laterally, and exaggerated, reflecting the hyperdynamic LV motion. Left atrial pressure (and pulmonary capillary wedge pressure) is elevated by the regurgitant flow, with an associated systolic V wave, though its height is not a reliable measure of the severity of MR. The left atrium and the diastolic size of the left ventricle are enlarged. As pulmonary venous pressure becomes elevated, dyspnea or even pulmonary congestion may occur. In the later phase, pulmonary hypertension may develop, causing pulmonary artery dilation, right-sided heart failure, and systemic venous congestion.
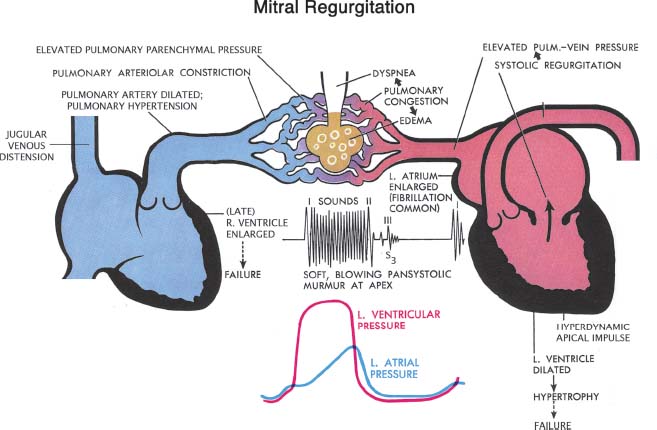
FIGURE 34.2 Schematic diagram representing pathologic complications of chronic MR. (Netter illustration adapted from www.netterimages.com. © Elsevier Inc. All rights reserved.)
Untreated chronic mitral valve abnormalities (including regurgitation or stenosis) often lead to a common endpoint, with left atrial enlargement, pulmonary hypertension, AF, and left-sided congestive heart failure. If the situation is not corrected, it may progress to include left atrial thrombosis, hemoptysis, and right-sided heart failure.7 Cardiac output is usually normal in the early phases, but may be reduced in the later phases of the disease if the MR goes untreated.
The natural history of chronic MR may involve many years of being asymptomatic, the so-called compensated phase of MR. In early years, chronic MR leads to increased LV size and mass and increased LV end-diastolic volume associated with a normal or elevated ejection fraction. At this stage there are few or no symptoms, because the dilated and compliant left atrium and LV allow accommodation of the regurgitant volume at normal filling pressures. However, as these mechanical changes progress, LV contractile dysfunction and hemodynamic derangements begin to occur, including pulmonary congestion, heart failure symptoms, and increases in LV end-systolic and end-diastolic diameter. Reduced forward flow is a very late occurrence.
The chest x-ray often shows left atrial enlargement with a double-density and widened carina, with no other findings early in the course of chronic MR. Later, the left ventricle dilates and there may be signs of pulmonary venous congestion. The lateral chest x-ray may show posterior protrusion of the left atrial cavity, a prominent pulmonary trunk, or small pleural effusions.
After clinical evaluation, a transthoracic echocardiogram (TTE) is useful for determining the MR severity, mechanism, etiology, presence of flail, LV size and function, left atrial size, abnormalities of other valves, and right ventricular (RV) systolic pressure (Table 34.3). It is also useful for assessing serial changes in LV size and function and evaluating the patient after a change in symptoms. An exercise echo is often useful for determining the change with exercise in LV size, LV function, or RV systolic pressure, and for discovering inducible symptoms or arrhythmias. Occasionally a transesophageal echocardiogram may be needed to better assess the severity and etiology of MR (Table 34.4). An exercise echocardiogram is often useful for determining the severity and effect of the disease on the patient’s exercise hemodynamics. It helps to detect latent myocardial dysfunction, and inducible problems not present at rest including pulmonary hypertension, arrhythmias, symptoms, and decreased ejection fraction. Cardiac catheterization for left ventriculography (Fig. 34.3) and hemodynamic measurements is helpful in rare specific conditions. Magnetic resonance imaging may also be of value, but its routine use in management of patients with chronic MR is unproven.
TABLE
34.3 Indications for Transthoracic Echocardiography in MR

From Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol.2006;48:e1–e148, with permission from Elsevier.
TABLE
34.4 Indications for TEE in MR
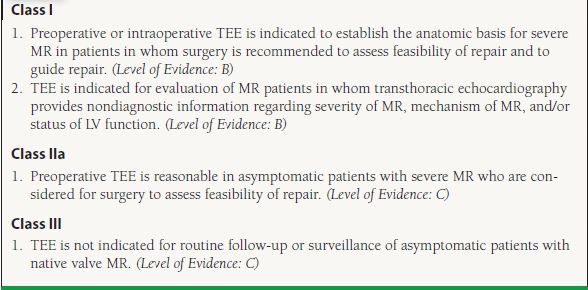
From Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48: e1–e148, with permission from Elsevier.
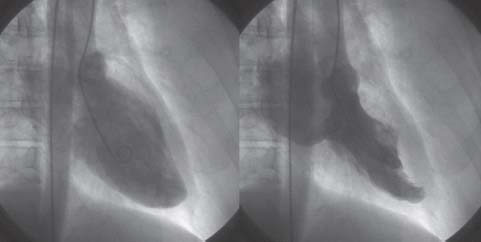
FIGURE 34.3 Left ventriculogram in the RAO projection in diastole (left) and systole (right), showing severe MR that completely fills the left atrium.
MECHANISM OF MITRAL REGURGIATION
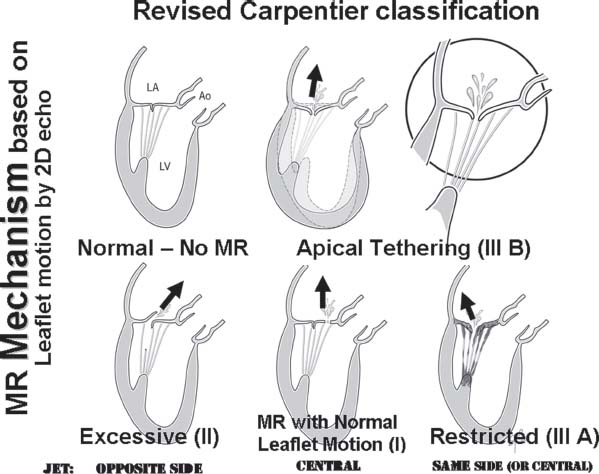
The mechanism of MR can be determined by echocardiography by looking at leaflet motion and color Doppler jet direction.8 First, the patient’s leaflets are categorized by 2-D echocardiography into those with normal, excessive, or restricted motion. Then additional information is gained by looking at the location, and direction of the regurgitant jet by color Doppler.
Mitral valve disease may have numerous causes, including myxomatous degeneration, ischemic, rheumatic, congenital, endocarditis, autoimmune disorders, and post radiation valve lesions. In the last half-century, there has been a remarkable increase of the frequency of myxomatous degeneration in surgical populations, with a decline in postinflammatory (rheumatic) cases, while ischemic- and infective endocarditis–associated MR have continued at a relatively low frequency.
In myxomatous degeneration, the most significant abnormality is abnormal elasticity of various portions of the mitral valvular apparatus, including the mitral annulus, chordae tendineae, and leaflets. There may be a variable amount of redundancy and enlargement of the leaflets (which, in the extreme is called Barlow syndrome) and chordae (sometimes called fibroelastic deficiency). In long-axis views, it is easy to see which leaflet is moving into the atrial side of the coaptation line in systole, indicating prolapse or flail. Additionally, the direction of the regurgitant jet provides supplemental diagnostic information (Fig. 34.4). In patients with excessive leaflet motion, the jet is directed to the opposite side of the most affected leaflet. Intercommissural views (transthoracic apical two-chamber and midesophageal transesophageal views aligned parallel to the intercommissural line at about 60 degrees multiplane angle) are useful for determining which portion of a leaflet is abnormal. Parasternal short axis and the “surgeon’s view” from the left atrium by three dimensional (3-D) echo also is helpful in appreciating the exact location of various lesions (Fig. 34.5) and differentiating the presence of multiple mitral abnormalities.
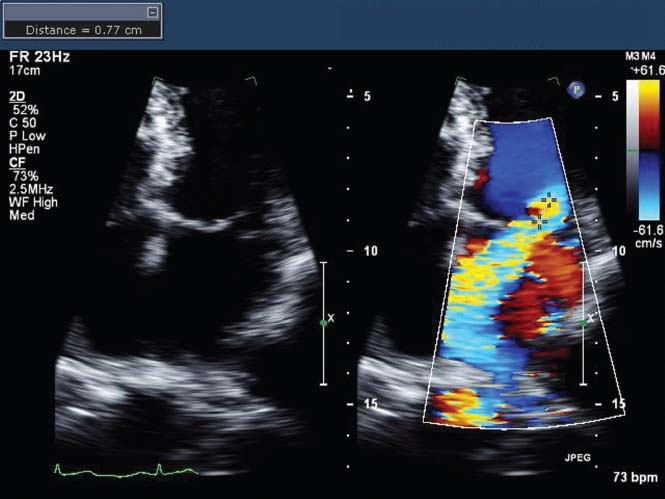
FIGURE 34.4 Transthoracic apical four-chamber 2-D echo image (left) and color Doppler image (right) in a patient with posterior leaflet flail. Note that the MR jet is deflected to the opposite (anteromedial) side of the left atrium by the unsupported posterior leaflet. Also note the flow convergence on the LV side of the jet, with a measured aliasing radius of 0.77 cm, from which a ROA of 0.46 cm2 was calculated, based on a measured MR maximum velocity of 505 cm/s.
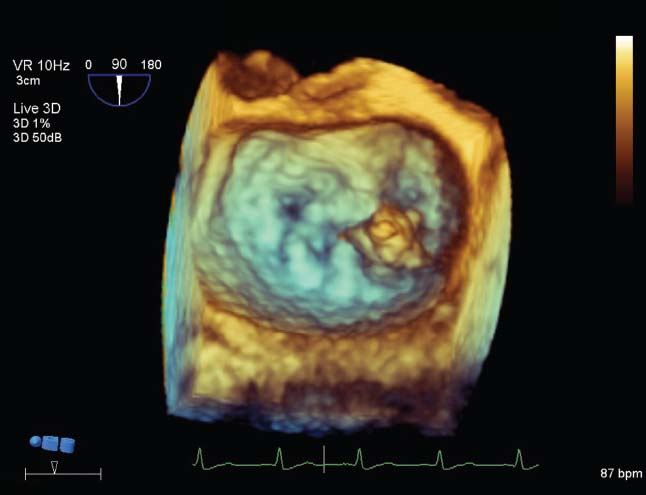
FIGURE 34.5 Transesophageal 3-D echo image using the “surgeon’s view,” showing that the morphology of the mitral valve causing regurgitation involved a flail portion (possible vegetation) located at the medial commissure (to the right on the image).
Rheumatic heart disease is characterized by leaflet thickening, diastolic mitral doming, valvular and subvalvular fibrosis, and various degrees of systolic and diastolic restriction of leaflet motion. In most cases of restriction involving both leaflets, the jet of MR is central. In some patients, the posterior leaflet is more restricted and the jet direction is posterior.
Ischemic MR most commonly represents “functional MR,” which results from remodeling, enlargement, and “sphericalization” of the left ventricle. Functional MR from nonischemic cardiomyopathy is very similar. Both are caused by “apical tethering” of normal leaflets (Fig. 34.6). The length of mitral tissue, including the leaflets, chordae, and papillary muscle, is fixed. As the left ventricle dilates, often after myocardial infarction, the LV wall and papillary muscles are displaced outward, which tethers all or part of the mitral leaflets downward away from the left atrium, reducing the amount of leaflet tissue available for coaptation. In many cases, the result is MR, with a central, or in some cases, a posterior jet direction. In rare cases, a focal infarction may cause elongation or disruption of the papillary muscle, leading to excessive leaflet motion (often involving both leaflets) most commonly the medial side of both leaflets from medial papillary muscle abnormalities.
FIGURE 34.6 Artist’s renderings of the mechanisms of MR defined by determination of leaflet motion by echo imaging (similar to the Carpentier classification (I, II, IIIa, and IIIb) and determination of jet direction using color Doppler. Apical tethering, often from ischemic heart disease, due to LV enlargement (Carpentier Class IIIb) is often called “functional” MR. Though the annulus may be dilated in proportion to the ventricular enlargement, the primary problem is the ventricle, not the annular dilation.
Mitral valve endocarditis can cause leaflet or chordal disruption, flail, and perforations. These often cause MR with associated nodular hypermobile densities (vegetations) that are the echocardiographic hallmarks of the disease. Care should be taken to look at adjacent valves and the perivalvular tissue looking for abscess formation or paravalvular leakage. MR secondary to the phospholipid antibody syndrome (the Lupus anticoagulant) is associated with symmetric thickening of leaflets, noninfected vegetations, and, at a later stage of the disease, fibrosis and leaflet restriction.
QUANTITATION OF MITRAL REGURGITATION
MR may be quantitated using a variety of echo and Doppler methods, including spatial mapping, flow convergence, pulmonary vein velocity patterns, vena contracta width, continuous-wave Doppler density and shape, and quantitation of antegrade valvular flow volumes. It is best to use a “weighted average” of multiple methods, emphasizing more the methods that have good-quality data in that patient.9
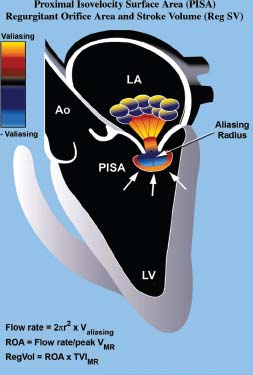
The flow convergence method, also called the proximal isovelocity surface area (PISA) method, involves assessing the color images on the LV side of the MR (Fig. 34.7).10
FIGURE 34.7 Method of quantifying the severity of MR using the PISA method (see text for details). (Adapted from Savage RM, Aronson S, Thomas JD, et al. Comprehensive Textbook of Intraoperative Transesophageal Echocardiography. Lippincott Williams & Wilkins. Chapter 28, 2005:512.)
This flow convergence zone is the location where the blood is accelerating, as the flow stream narrows progressively to the regurgitant orifice, where there is a pressure drop from LV pressure to left atrial pressure. Color Doppler tracks the location of increases in velocity on the LV side of the regurgitant orifice and shows this as color aliasing in the proximal convergence zone. When the shape of the aliasing contour is hemispheric, one can calculate the surface area of the aliasing hemisphere from the radius of the hemisphere. Flow can therefore be calculated from the aliasing velocity (v), extracted from the “color bar” (reflecting machine settings), and the measured radius (R) measured from the center of the jet to the transition of color aliasing. When this is combined with the maximum velocity, the maximum systolic area of the regurgitant orifice can be calculated according to the formula 2πR2v divided by maximum systolic velocity through the valve (Vmax) obtained by continuous-wave Doppler. The advantage of this formula is that it calculates the actual size of the regurgitant lesion, a fundamental parameter of valve integrity, which is less load dependent than other parameters. A maximum instantaneous regurgitant orifice area (ROA) > 0.4 cm2 is indicative of severe regurgitation, whereas <0.2 cm2 is considered mild.
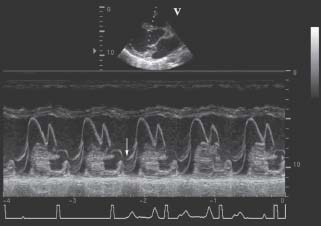
Pulmonary vein flow profiles (Fig. 34.8) are also useful for determination of severity of MR.11 The normal pulmonary vein pattern includes a velocity during ventricular systole that is higher than antegrade velocity during diastole, a pattern that persists in mild and sometimes moderate MR. When the regurgitation is moderate or moderately severe, the systolic velocity of pulmonary vein flow becomes blunted, with systolic velocity less than diastolic velocity. With even more MR, there is cessation of systolic flow. In patients with severe MR, in one or the other pulmonary vein profile, there is often reversal of systolic flow, with retrograde flow occurring, away from the left atrium during ventricular systole. This occurs because of the large V wave during ventricular systole in the left atrial pressure, which transiently becomes higher than pulmonary parenchymal pressure.
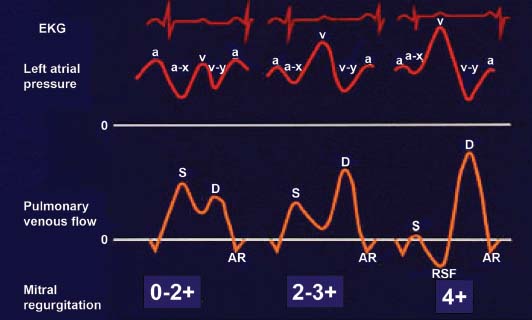
FIGURE 34.8 Stylistic ECGs, left atrial pressure waveforms, and pulmonary vein pulsed Doppler velocity recordings, in three patients: normal or mild or moderate MR (on the left), moderately severe or 2 to 3+ MR (in the middle); and severe, 4+ MR (on the right). Note the reversal of systolic flow (RSF) associated with severe MR, which results from the systolic V wave in the left atrial pressure. (Adapted from Klein AL, Obarski TP, Stewart WJ, et al. Transesophageal Doppler echocardiography of pulmonary venous flow: A new marker of mitral regurgitation severity. J Am Coll Cardiol. 1991;18:518–526.)