Fig. 10.1
Blood pressure drop in the vascular bed, from large, conductance arteries to veins (Redrawn from Ref. [1]). MAP mean arterial pressure, VP venous pressure
In the different forms of experimental hypertension, an increased resistance to flow was actually observed at the microcirculatory level [3]. Structural, mechanical and functional changes in small vessels may determine a reduction of the internal lumen and, therefore, an increase in vascular resistance. This may directly induce an increase of systemic blood pressure. There is general agreement on the fact that essential hypertension is associated with the presence of structural alterations in the microcirculation [1–5]. An increase in the vascular wall thickness, together with a reduction in the internal diameter, may play a major role in the increased peripheral resistance, being also a possible adaptive mechanism to the increased blood pressure load.
The mechanisms underlying the development of microvascular structural alterations are only partially elucidated [6–9]. However, if we recognise that structural alterations might have hemodynamic consequences, then their evaluation represents an important target, also in terms of cardiovascular risk stratification [8, 10].
10.2 Methods of Evaluation of Vascular Structural Alterations
The methods for the evaluation of microvascular structural alterations available in humans are relatively few (Table 10.1).
Table 10.1
Advantages and disadvantages of the commonly used techniques for assessment of functional and remodelling of small arteries
Technique | Advantage | Disadvantage |
|---|---|---|
Histology | Easy to analyse; sample can be stored; inexpensive | Microinvasive; artefacts introduced by fixation, staining and dehydration; possible coarctation of the samples |
Plethysmography | Easy access; inexpensive; evaluation of minimum vascular resistance in the forearm; drug challenge | Challenging to have good performance; need for standardisation, personnel training and setup |
Wire and pressure micromyography | Well-standardised technique, evaluation of vascular morphology and function; evaluation of parameters with prognostic significance (media to lumen ratio) with high sensibility, specificity and accuracy; reliable and reproducible functional data (better evaluation with pressure micromyography) and morphological data (more precise with wire micromyography) | Microinvasive; well-trained personnel; bias related to mechanical damage of the vessel |
Scanning laser Doppler flowmetry (retinal district) | Non-invasive; easy access to vascular district; correlates with parameters obtained with micromyography | Not extensively used; further validation and standardisation of the technique needed |
Histological approaches are charged with substantial pitfalls, due to the artefacts introduced by fixation, staining and dehydration with possible coarctation of the samples [1] (Table 10.1).
The methodological approach that has the widest application is represented by the wire or pressure micromyography that allows a reliable evaluation of structural changes within the vascular wall and of functional aspects as well (Table 10.1).
Wire micromyography (Fig. 10.2) was developed by Mulvany and Halpern [11, 12] in the 1970s, and it was subsequently applied to vascular segments (mesenteric, cerebral, coronary, renal, femoral, etc.) obtained from several animal models of hypertension. Interestingly enough, the wire micromyography technique was also used for the evaluation of morphology and function of small arteries obtained from biopsies of the subcutaneous tissue from the gluteal or anterior abdominal region, in normotensive subjects as well as in hypertensive patients [13, 14].
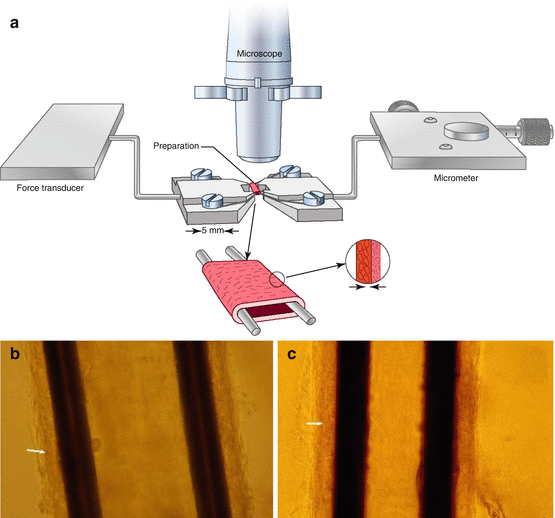

Fig. 10.2
Wire micromyography: drawing (a) and photo of subcutaneous small resistance arteries mounted on a wire micromyograph as a ring preparation (Mulvany’s technique) (b, c). (c) Small artery of a normotensive subject. (b) Small artery of a hypertensive patient; the internal lumen is smaller and the media thickness slightly greater. The arrow indicates the boundary between the tunica media and the tunica adventitia. Vertical black areas: stainless steel wires used for cannulation
For this technique, small artery segments, diameter ranging from 100 to 300 μm, few millimetres long are obtained by dissection and made free of periadventitial fat tissue. Then the vessels are cannulated with stainless steel wires (40 micron of diameter), resulting in a ring preparation that is mounted on a micromyograph. A mechanical stretch may be applied through a micrometric screw while a force transducer records the passive tension developed, or the vessel may be maintained to a constant stretch and stimulated to contract by adding various substances to the organ bath, such as norepinephrine, potassium, serotonin, etc., thus allowing the measurements of the active tension developed. It is mandatory that the vessel is not damaged during dissection, cannulation and mounting procedures, inasmuch even minimum damage to the vessel’s wall may translate into alterations in the contractile responses and in errors in the morphological evaluations as well.
Subsequently, the vessel, in a relaxed condition, is transferred on the stage of an immersion lens microscope, and through a micrometric ocular (magnitude about 600×), the total wall thickness as well as the relative thicknesses of the adventitia, media and intima tunica and the internal diameter are evaluated. Also cross-sectional areas and volumes may be calculated, together with the thicknesses of the different tunicae in normalised conditions; that is to say these thicknesses would be present in vivo with the vessels distended by a transmural pressure slightly less than 100 mmHg. It is therefore possible to obtain reliable and reproducible morphological data, overcoming many of the limitations related to histomorphological approaches [1].
The most relevant and useful parameter obtained with micromyographic approaches is represented by the tunica media to internal lumen ratio, since it appears independent from the vessel’s dimensions [15, 16].
On the contrary, media thickness and internal diameters are obviously dependent on the dimensions of the vascular segment [15]; therefore comparisons between such parameters examined in different experiments may be problematic in the absence of a rigorous selection of the sampling site, which is not feasible in humans and sometimes difficult also in experimental animals.
Possibly, small mesenteric arteries of the rats represent the vascular district where it is possible to obtain vascular segments from anatomically well-defined positions: here the branching pattern allows an easy identification of small arteries of 1st, 2nd, 3rd and 4th level, until they enter the intestinal wall as arterioles with diameters of around 80 μm.
A possible alternative to the wire micromyography is represented by perfusion-pressure micromyography (Fig. 10.3). With this approach, isolated vessels are mounted in a pressurised myograph chamber and slipped into two glass microcannulae, connected to a perfusion system that allows a constant intraluminal pressure of 60 mmHg [17]. Morphology of the vessels is evaluated by computer-assisted video analysers. In a previous study, no difference was observed between wire or pressure micromyography in terms of morphological evaluations, provided that the vessels are analysed in comparable conditions [15, 18–20]. Vessels may be analysed at constant pressure or constant flow. It is likely that, when compared with wire micromyography, pressure micromyography allows a better evaluation of functional responses, while the precision of the morphological assessment could be slightly lower [15].
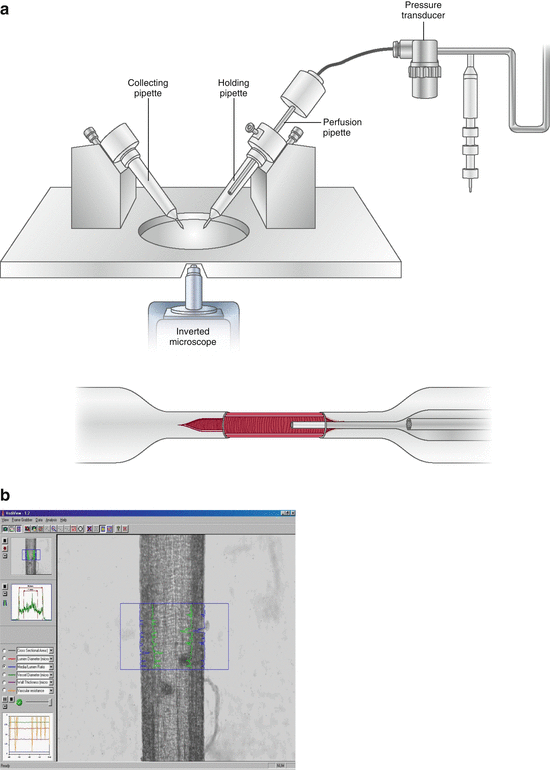

Fig. 10.3
Pressure micromyography: drawing (a) and image of a mounted vessel during measurement of vessel wall thickness (b)
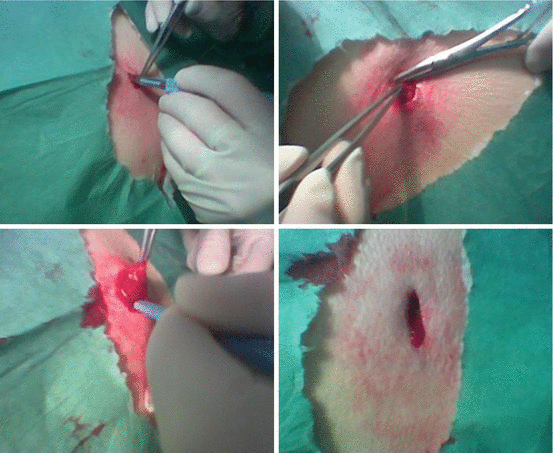
In a group of normotensive subjects and hypertensive patients, a close correlation between media to lumen ratio of subcutaneous small arteries, measured by wire micromyography, and forearm minimum vascular resistance, measured by plethysmography [14], was observed (see dedicated chapter). Thus, although the two methods seem to possess different characteristics (a direct, invasive in vitro ex vivo approach and an indirect, non-invasive in vivo technique), results obtained are in reasonable agreement. In summary, micromyographic in vitro studies have the advantage to allow a precise and accurate measurement of morphological features of small vessels, without the fixation artefacts related to histological studies, but have also the limitation of the relative invasiveness of the procedure. In fact, dissection and mounting of the single vessel on the pressure or wire micromyograph require the availability of adequate tissue (in humans usually obtained during a surgical procedure or by a biopsy of the subcutaneous fat tissue) [13, 14]. These subcutaneous or skin biopsies are small, ethically acceptable interventions which are tolerated well by patients and have been employed already for some time to study the structure of small arteries from hypertensive patients [18]. Biopsies of the gluteal region have been performed in two essentially equivalent ways [18]. After local anaesthesia has been administered, the classical way to perform these biopsies is to obtain a segment of skin from the upper external quadrant together with subcutaneous fat (Fig. 10.4). This allows vessels to be visualised from the inside of the skin, isolated from the subcutis as well as from the underlying subcutaneous fat [18]. An advantage is that the vessel diameter can be grossly evaluated and essentially equivalent vessels may be isolated from different patients, facilitating comparison of parameters since the arteries obtained are from essentially the same level of the vasculature. Another technique, used by Schiffrin and coll. [18], is simply to cut a horizontal incision 1 cm in length in the upper external gluteal quadrant. The superficial subcutaneous tissue and immediately underlying fat are excised carefully with a scalpel. No skin is removed, facilitating repeat procedures and rendering the intervention a smaller, less invasive and better tolerated one, with very little residual discomfort and practically no opportunity for complication [18]. A disadvantage of this approach is that it is not known whether exactly comparable vessels have been dissected [18].