Biopsy
Explanted heart
Clinical autopsy
Medico-legal autopsy
Diagnosis of myocarditis and cardiomyopathies
Diagnosis
Correlation with imaging techniques
Cause of death
Diagnosis of rejection, infection, or lymphoproliferative disorder in a transplanted heart
Effect of a treatment
Contribution to the death
Extension of disease
Detection of hereditary diseases
Malpractice claims
Presently, the largest number of histopathological studies on the whole heart is carried out in forensic investigation due to the following reasons:
1.
Cardiac pathology is responsible for 80 % of the sudden deaths that require a forensic autopsy to discard a violent cause of death.
2.
Cardiac pathology can be a coadjuvant factor for deaths that occur during extracardiac surgical interventions or during hospital stays; situations where medical malpractice claims are frequent.
3.
Cardiovascular surgical procedures such as correction of congenital defects, catheterizations, ablations, pacemaker implantations, prostheses, coronary by-pass grafts, etc., can result in fatal outcomes that also require legal investigation.
4.
Cardiac pathology can be responsible for traffic-related accidents, or work or home-related accidents, which cause death due to traumatic lesions.
In the context of sudden death, it is also essential to study the heart in order to identify potential hereditary diseases, and through this offer counselling and protect other family members. Logically, before performing an autopsy on the heart it is necessary to have as much information as possible regarding: family and personal history, previous cardiovascular events, surgical interventions and circumstances of the death (whether witnessed, related to physical activity, duration and type of resuscitation manoeuvres attempted).
1.2 Study of the Heart
The study of the heart begins with the removal of the sternum and the inspection for possible intrapericardial effusion (quantity and characteristics: haematic, serous, purulent); or mediastinal haemorrhage due to rupture of the extrapericardial aorta. If gas embolism is suspected, the pericardial sac must be opened under water to check for leakage of gas bubbles through an incision on the pericardial surface. Once the pericardial sac is opened, and to discard thromboembolism, the trunk of the pulmonary artery can be sectioned 2 cm above the pulmonary valve, although many authors recommend for this purpose the opening of the pulmonary arteries from the posterior side. In cases of aortic dissection, the aorta should be examined along its entire length to look for a tear in the intima, and also in the external wall in cases of cardiac tamponade or mediastinal or retroperitoneal haemorrhage.
The heart must be removed as a whole by cutting the inferior vena cava close to and above the diaphragm; the pulmonary veins after checking their correct drainage in the left atrium; and the aorta and pulmonary trunk 2 cm above the semilunar valves.
Once the heart has been removed it should be washed to eliminate blood clots, and weighed. The heart weight must be compared with the expected weight for the gender and body weight. It is estimated that there is an increase of 5 % in the weight of a fixed heart in formalin. It can also be useful to measure the ventricular axes in the posterior surface of the heart, taking as a reference the crux cordis (indicative of the presence of dilation and/or ventricular hypertrophy). A transversal axis of 10–11 cm, and a longitudinal axis of 9–10 cm, is considered normal. Next, a macroscopic study is initiated, which is summarised in Table 1.2. The Annex at the end of this chapter provides the normal reference values for cardiac dimensions and weight. It is recommended that photographs of any lesions are taken, or even the absence of alterations, in specific legal cases.
Table 1.2
Gross examination of the heart
External examination | Shape: Normal, globoid, ventricular aneurysms |
Pericardium: Roughened or nodular appearance, whitish plaques | |
Epicardium: Quantity and distribution of epicardial adipose tissue | |
Ventricles | Wall thickness: concentric or asymmetric hypertrophy |
Subaortic septum: Sigmoid, whitish endocardium | |
Ventricular lumen: Diameter, presence of mural thrombi | |
Myocardial lesions: Scars, recent necrosis, haemorrhage, disarray, mottled appearance, adipose infiltration, location (basal mid-ventricular, apical) and distribution (subendocardial, transmural, subepicardial) | |
Valves | Tricuspid: Perimeter, vegetations, myxoid changes |
Pulmonary: Number of leaflets, vegetations, dysplastic leaflets | |
Mitral: Perimeter, commissure fusion, prolapse, annular calcification, vegetations, shortening and fibrosis or rupture of the tendinous cords | |
Aortic: Perimeter, number of leaflets, calcifications in the sinuses or in the sinotubular junction, commissure fusion, stenosis, vegetations, aneurysms, dysplastic leaflets | |
Coronary arteries | Ostia coronaria position |
Course | |
Permeability (degree of luminal reduction) | |
Dominance | |
Atria | Foramen ovale: patency, aneurysms |
Interatrial septum thickness | |
Mural or appendage thombi | |
Ascending | Atheromas |
Dilatations, aneurysms | |
Aorta | Intimal tears in dissections |
Orifices of coronary bypass grafts |
The study of the heart begins with an external examination to assess its shape, appearance of the pericardium, absolute mass of epicardial adipose tissue, etc. (Fig. 1.1).
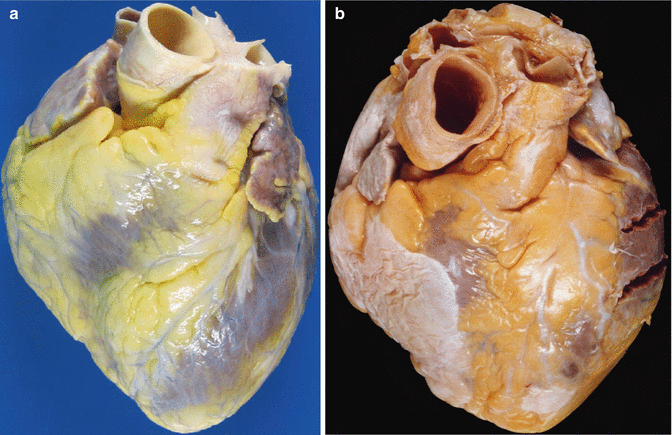

Fig. 1.1
(a) Normal heart with epicardial adipose tissue distributed along the AV groove and in the course of the anterior descending coronary artery. (b) Whitish patches (Soldier’s patches) in visceral pericardium classically attributed to mechanical trauma or healed pericarditis
There are several methods to open and dissect the heart depending on the pathology to be studied but, taking into account that in the majority of forensic cases there is no medical antecedents or clinical records of the deceased, the method of choice to study the heart must be the one that allows the diagnosis of the most frequent diseases. The classical method of dissection following the direction of the blood flow can be used in cases of congenital heart disease in children, but not for the study of adult heart pathology (myocardial infarction, cardiomyopathies, infiltrating diseases, etc.) in which several parameters such as the presence of myocardial lesions, wall thickness and ventricular size need to be evaluated. For this reason the most convenient method of dissection of the adult heart involves sectioning the ventricular cone into slices of 1–2 cm in thickness, parallel to the posterior atrioventricular groove, starting from the apex to the base of the papillary muscles. Using this method, about four or five biventricular slices are obtained and the cardiac base is left intact (Fig. 1.2). This sectioning is best performed on a fixed heart and with long-bladed knives.
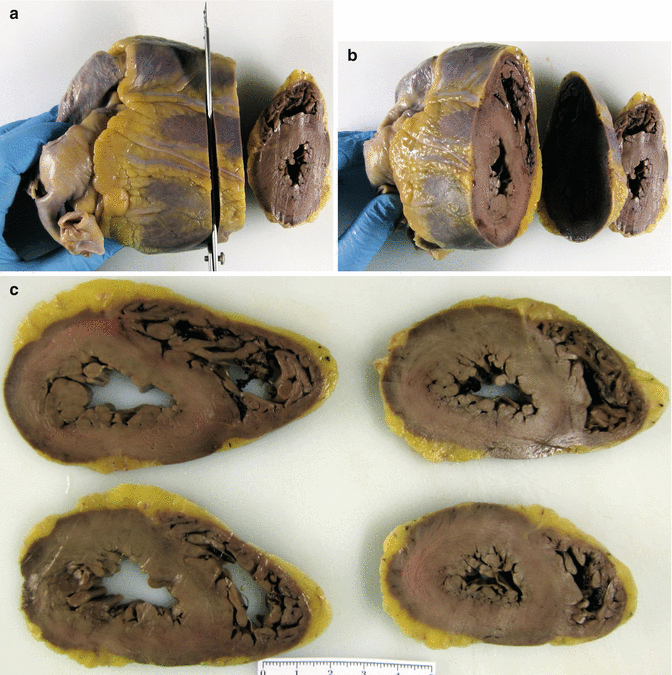

Fig. 1.2
(a–c). Protocol to study the heart by transversal sectioning from the apex to the papillary muscles, obtaining a closed base with intact valves and four to five biventricular slices
1.2.1 Study of the Myocardium
The location and distribution of myocardial lesions, together with the diameter of the ventricles, should be evaluated in the sliced biventricular sections. The anterior wall of the LV spans from the anterolateral papillary muscle to the septum; the lateral wall is situated between the two papillary muscles; and the posterior/inferior LV wall is adjacent to the posterior papillary muscle (Fig. 1.3). Pathologists maintain the posterior wall terminology, whereas clinicians, in keeping with the heart anatomical position, prefer to name it inferior wall.
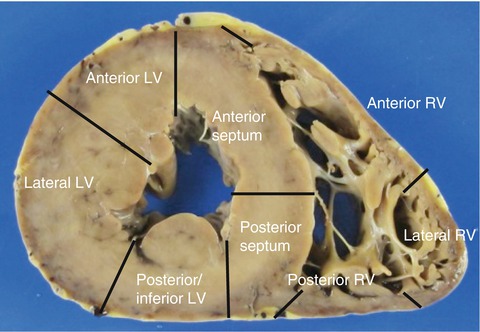

Fig. 1.3
Nomenclature of the ventricle walls
The mid-ventricular slice should be used to measure the wall thickness and the diameter of the LV, excluding the trabeculae and papillary muscles (Fig. 1.4). A possible dilation of the RV should be evaluated in the cardiac apex. The thickness of the anatomical sections is not equivalent to those obtained by echocardiography since this is performed with the heart in telediastole. Values considered normal are 10–15 mm for the thickness of the LV and the septum, and 3–4 mm for the RV, during necropsy. Larger values are indicative of cardiac hypertrophy; however, diagnosis of hypertrophy should be based chiefly on the weight of the heart relative to that expected for the body weight.
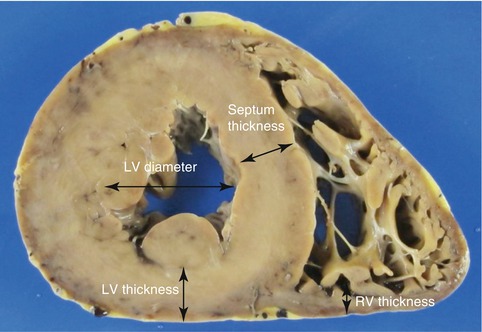

Fig. 1.4
Measurement of the ventricular thickness and the diameter of the LV, excluding the papillary muscles and trabeculae. The thickness of the RV is measured at its posterior wall since the anterior wall presents more epicardial adipose tissue and adipose infiltration, especially in elderly people
1.2.2 Study of the Cardiac Base and Valves
The cardiac base is opened following the direction of blood flow, starting in the right atrium that is opened from the inferior vena cava to the apex of the right atrial appendage, to preserve the sinoatrial node of the conduction system (Fig. 1.5) (see Chap. 10). Then examine the tricuspid valve through which, in normal conditions, three average-sized fingers can be placed.
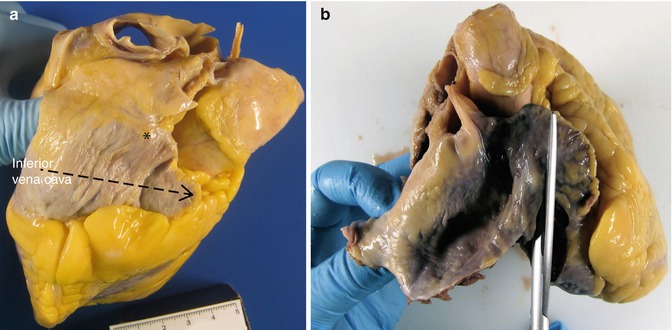

Fig. 1.5
(a) The discontinous arrow points to the direction of the cut to open the right atrium. The asterisk indicates the position of the sinoatrial node. (b) Opening with scissors
Then, open the lateral border of the RV (Fig. 1.6a) and inspect the opened tricuspid valve, the right atrium and the basal portion of the RV. The perimeter of the valve is measured at the level of the annulus (Fig. 1.6b).
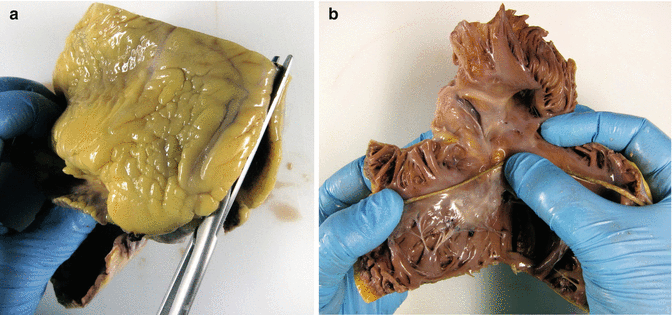

Fig. 1.6
(a) Opening of the lateral border of the RV. (b) Measurement of the tricuspid valve perimeter with the aid of a length of string
The most important structures of the right side of the heart are compiled in Fig. 1.7. The AV node is positioned in front of the mouth of the coronary sinus (see Chap. 10). The tricuspid valve has one large anterior papillary muscle, a septal muscle (Lancisi’s muscle) which can be double or multiple, and a posterior papillary muscle formed by several smaller ones. The septomarginal trabecula (also known as moderator band) extends from the inferior part of the septum (continuation of the septal band of the supraventricular crest) to the base of the anterior papillary muscle at the tricuspid valve. This is the inferior limit of the inflow tract of the RV and through its interior runs the continuation of the right bundle of the conduction system.
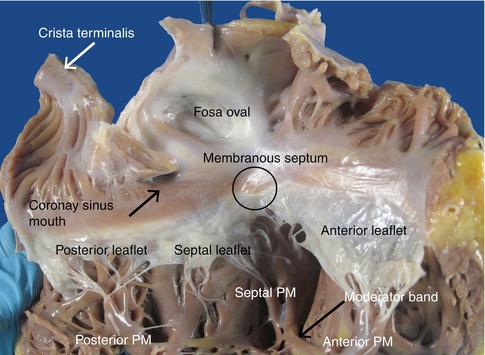

Fig. 1.7
View of the right chambers after opening through the lateral border. The most relevant structures are indicated
In one third of adults, the foramen ovale does not close correctly (Patent foramen ovale) and functions as a competent flap valve, ensuring that the orifice remains closed since greater pressure exists in the left atrium (Fig. 1.15).
After, following in the direction of the blood flow, open the outflow tract of the RV and the pulmonary artery (Fig. 1.8). The annulus of the pulmonary artery is located 1.5 cm above the annulus of the aortic valve and has three leaflets: the right leaflet is located near to the aortic valve right leaflet, and the left leaflet is found near the aortic valve left leaflet (Fig. 1.9). The valve perimeter is measured at the sinotubular junction.
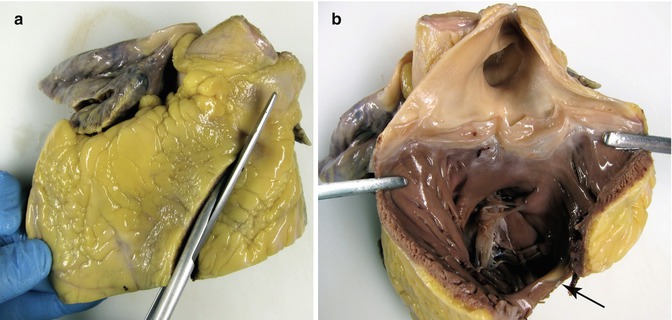
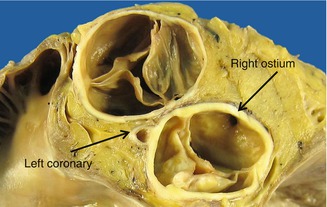

Fig. 1.8
(a) Sectioning through the RV outflow tract. (b) View of the opened pulmonary valve and the origin of the pulmonary arteries. In the bottom, the moderator band can be observed (arrow). In some cases the pulmonary valve can have four leaflets (see Chap. 7)

Fig. 1.9
Transversal section at the level of the semilunar valves. At the top of the image, the pulmonary artery and, at the bottom, the aortic valve where the ostium of the right coronary artery and the origin of the left coronary artery trunk can be visualised
The opening of the LA is more arbitrary, generally between the left and right pulmonary veins, but attention must be paid to the atrial appendage in case possible thrombus is present (Fig. 1.10). View the mitral valve from the left atrium searching for prolapse, fusion of commissures, etc. (Fig. 1.11). It is estimated that two fingers can pass through the mitral annulus. Exceptionally, anomalous muscular bands can be found in the atria, similar to the false tendons of the LV (Fig. 1.12), which are associated in some cases with patent foramen ovale and to Chiari’s network, and can cause mitral valve insufficiency.
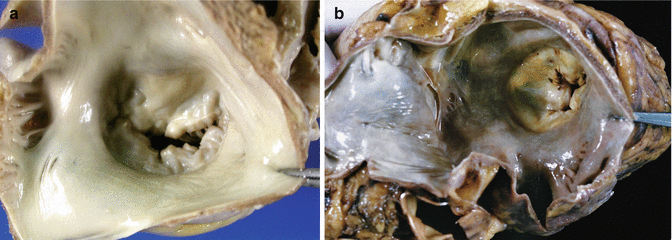
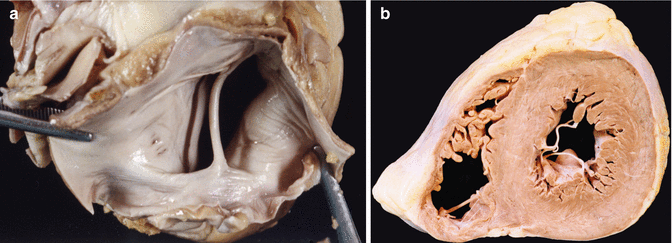

Fig. 1.10
Opening of the left atrium and appendage

Fig. 1.11
(a) A normal mitral valve as seen from the left atrium. (b) A rheumatic mitral valve: the atrium is dilated, with valvular fibrosis in the leaflets and commissure fusion

Fig. 1.12
(a) An anomalous band between the anterior and posterior wall of the left atrium. (b) False tendons between the papillary muscles and the septum, and in the RV
Next, open the LV laterally with a longitudinal cut between the papillary muscles (Fig. 1.13) to view the left atrium, the opened mitral valve (its perimeter is measured at the annulus level) and the left ventricle, paying special attention to the internal structure of the chambers and the mitral valve. The mitral valve consists of two leaflets: the anterior one is semicircular and forms part of the left ventricular outflow tract; the posterior leaflet has a scalloped appearance. The anterior papillary muscle is single whereas the posterior papillary muscle is usually double (Fig. 1.14). The area facing the LV is very trabeculated (1/4 of the total thickness).
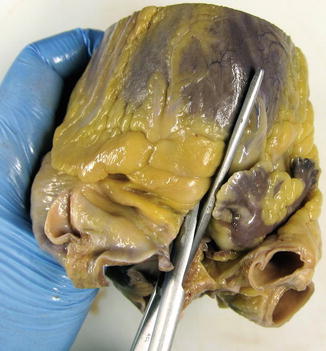
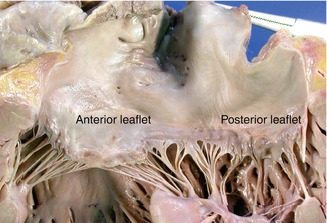
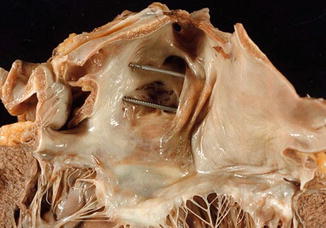

Fig. 1.13
Opening of the lateral border of the LV

Fig. 1.14
View of the opened mitral valve

Fig. 1.15
Patent foramen ovale as seen from the left side
Visual inspection of the aortic valve should be performed from above before its opening to determine the number of cusps, the presence of calcifications, commissural fusion, etc. (Fig. 1.16). Next, the outflow tract of the LV is opened by an oblique cut in the anterior wall proximal to the septum (Fig. 1.17) to inspect the ascending aorta (check for atheromas, aneurysms, intimal tears in aortic dissections, intramural haematomas, origin of coronary bypass, etc.) and the aortic root (consisting of the fibrous annulus, the sinuses of Valsalva and the sinotubular junction).
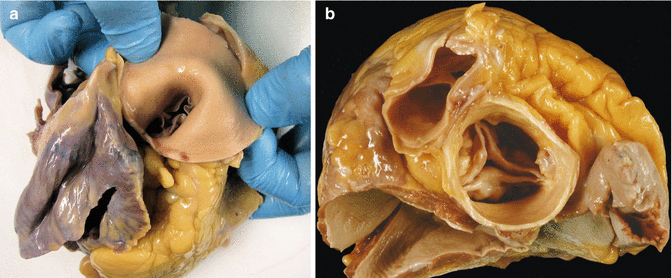
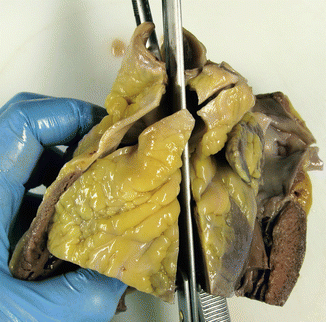

Fig. 1.16
(a) Inspection of the aortic valve before its opening. (b) Aortic valve with calcification in the three leaflets, in an 82-year-old woman with senile aortic stenosis

Fig. 1.17
Opening of the LV outflow tract. Long forceps serve as a guide
The aortic and mitral annuli are fused through the intervalvular fibrosa (fibrous continuity between the anterior leaflet of the mitral valve and the left and posterior sigmoids) (Fig. 1.18). The anterior Valsalva sinuses give rise to the coronary arteries (Fig. 1.9). Fenestrations are frequently found between the valve closure line and its free border (lunula) (Fig. 1.21a), and also fibrocartilaginous nodules (Arancio-Morgagni’s nodules) and papillary excrescences (Lambl’s excrescences) in the valve closure line (Fig. 1.19).
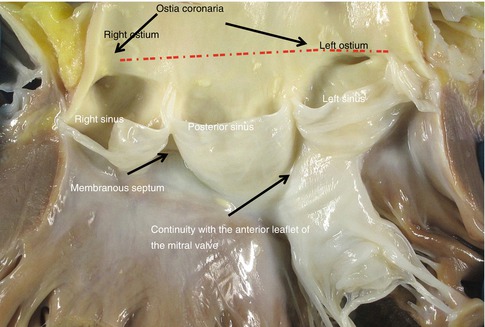
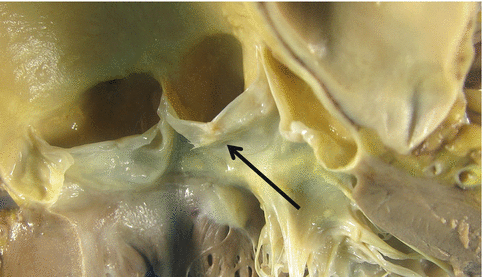

Fig. 1.18
Normal opened aortic valve where the Valsalva sinuses can be seen, with the origin of the coronary arteries (right (RC) and left (LC)). Discontinuos line = dashed line

Fig. 1.19
Fibrous nodules and Lambl’s excrescences in the valve closure line (arrow)
1.2.3 Study of the Coronary Arteries
The left and right coronary arteries arise from the corresponding aortic Valsalva sinuses, and their branches irrigate the entire heart. The most frequent coronary artery tree is depicted in Fig. 1.20. According to this tree, the posterior descending coronary artery originates from the right coronary artery (right dominance), although there are three possible types of dominance whose characteristics and frequency are described in Table 1.3.
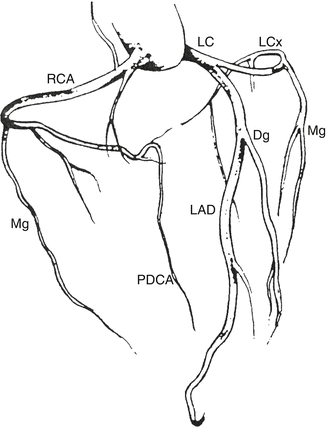

Fig. 1.20
Scheme of the coronary artery tree. RCA right coronary artery, PDCA posterior descending coronary artery, LC left coronary artery trunk, LAD left anterior descending coronary artery, Dg diagonal, LCx left circumflex, Mg marginal
Type I (77 %) (right) | The right coronary artery gives rise to the posterior descending coronary artery and ends: |
In the posterior interventricular groove without significant branches on the LV (5 %) | |
At distinct levels on the LV (55 %) | |
In the left cardiac edge with the inferior LV wall totally irrigated by the right coronary artery | |
Type II (8 %) (left) | The circumflex coronary artery gives rise to the posterior descending artery |
Type III (15 %) (balanced or codominance) | There are two posterior descending branches, one from each artery. In this type, the apex and the inferior ventricular septum rely on the left coronary artery since the LAD re-ascends through the posterior interventricular groove in one quarter or more of its length |
In one third of the population, the left main coronary artery is divided in three branches: the left anterior descending coronary artery (LAD), the circumflex coronary artery (Cx) and in between the intermediate branch (not shown in the figure). In these cases, the intermediate coronary artery replaces the first diagonal.
1.2.3.1 Study of the Origin of the Coronary Arteries
The study of the coronary arteries begins by examining their origin in the corresponding Valsalva sinuses. The diameter of the right coronary artery at its ostium is commonly slightly smaller than that of the left coronary artery ostium. There is usually one ostium in the sinus, in a central position, although in some cases variations occur that are considered normal: two or more ostia in the right sinus or two ostia in the left sinus (Fig. 1.21). When both coronary arteries arise from the same sinus, sudden death can occur (see Chap. 6). In up to 30 % of human hearts, the left anterior descending coronary artery penetrates the myocardial septum to 2–3 mm depth and for a length of 10 mm in the basal third portion (Fig. 1.22). When the depth is more than 4 mm, the permeability of the artery during contraction of the ventricle can be compromised, provoking myocardial ischaemia (see Chap. 6).
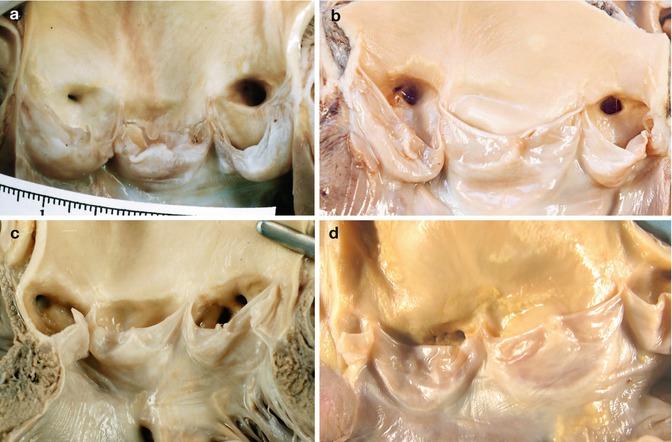
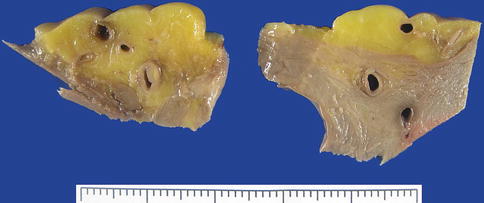

Fig. 1.21
Normal variations in the origin of the coronary arteries. (a) A unique ostium in each coronary sinus. The posterior leaflet is fenestrated. (b) Two orifices in the right sinus; the smallest one is the origin of the conus artery. This occurs in more than half of the hearts examined. (c) Independent origins of the left anterior descending and circumflex arteries in the left sinus (very infrequent). (d) In the right sinus three ostia can be recognized, corresponding to the right coronary artery, the conus artery and the right superior septal artery

Fig. 1.22
Intramural course of the left anterior descending coronary artery
1.2.3.2 Study of the Distribution and Permeability of the Coronary Arteries
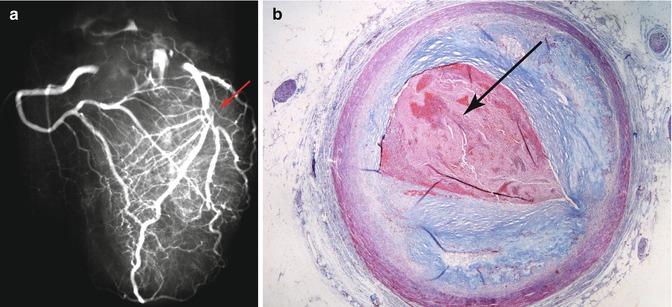
In cases of ischaemic heart disease, the ideal method is to perform radiography on the whole heart before sectioning, in order to identify calcified portions in the coronary arteries, possible stents, valve calcification, etc. It is also useful to carry out a post-mortem angiography by inserting contrast material through the origin of the coronary arteries, followed by radiography (Fig. 1.23) (the downside of this method is that the coronary ostia cannot be properly examined).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


