Fig. 28.1
Some self-expanding metallic airway stents in current use. Top left: a covered Ultraflex stent, showing the technique of grabbing the suture wound circumferentially around the proximal end of the stent. The suture material is now green in color, not black as shown here. Top right: an Alveolus AERO fully covered stent, half-deployed. The green marker corresponds to the proximal end of the stent in normal use. A blue suture is just visible through the external catheter at the proximal end of the stent. Bottom right: the deployment mechanism of the Alveolus AERO stent. After removing the white cardboard protector, the blue trigger is gradually moved proximally to deploy the stent. Bottom left: a part-deployed Micro-Tech stent, which also has a clear plastic outer sheath (Courtesy of Dr. Mark Slade.)
In common with several other SEMAS, the Ultraflex has nylon loops or sutures, tied with a knot, running circumferentially at each end of the stent. These can be grabbed with forceps to permit stent repositioning (see Figs. 28.1, 28.2, and 28.3). Until recently, the loop was the same color (black) as the rest of the stent, making its identification endoscopically challenging, but recently a green filament has been used.
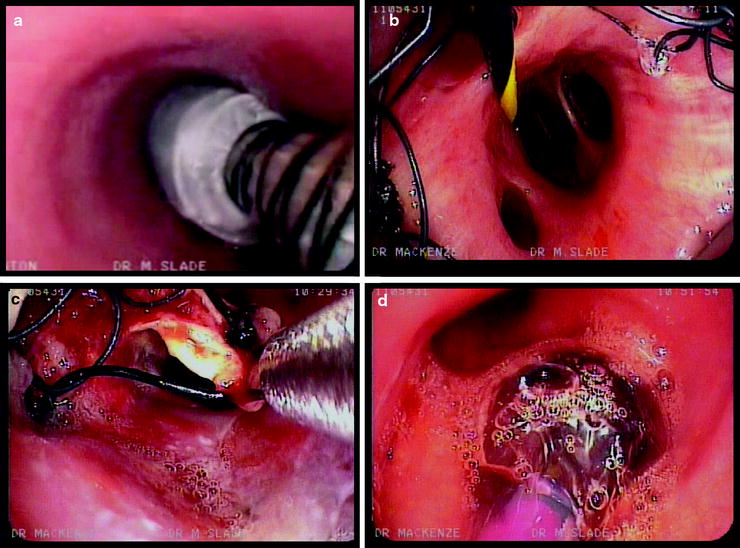
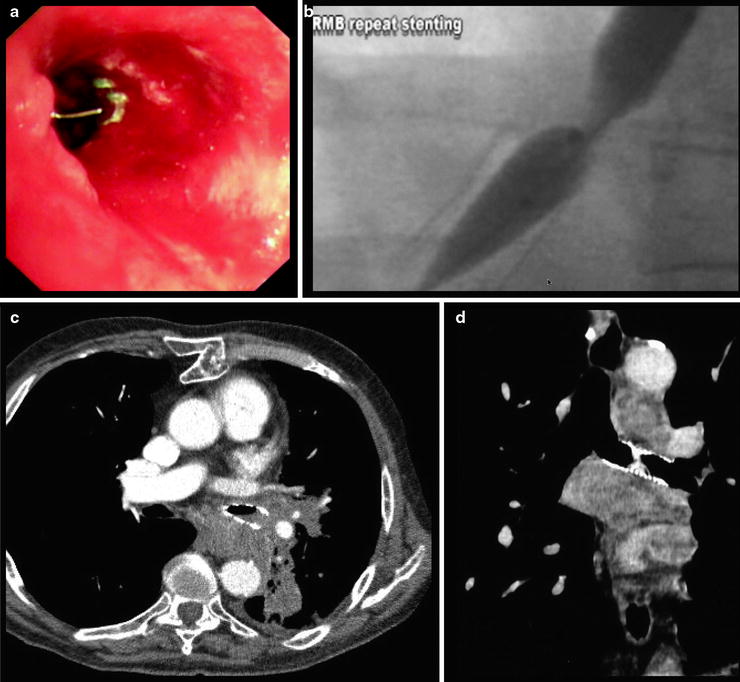

Fig. 28.2
Deployment and repositioning. Top left: a tracheal stent during deployment, illustrating endoscopic confirmation of stent positioning and distal to proximal release. Top right: confirming satisfactory distal position of a right main bronchial stent at middle lobe take-off. Bottom left: attempted distal repositioning of a stent by grabbing distal suture. Bottom right: post-deployment balloon dilatation of a stent in right main bronchus (Courtesy of Dr. Mark Slade.)

Fig. 28.3
Stent complications. Top left: a fractured strut of the proximal part of a left main bronchial stent is seen protruding into the bronchial lumen. This fracture was caused by an attempt to reposition the stent using forceps. The stent mesh was grabbed instead of the green suture, which is also just visible. Top right: a stricture has formed at the proximal end of an existing right main bronchial stent and is being ballooned. Bottom left: tumor ingrowth is visible into the distal end of a left main bronchial stent. Bottom right: a left main bronchial stent is obstructed by mucus impaction 6 weeks after placement (Courtesy of Dr. Mark Slade.)
Other stents use an external clear plastic catheter to compress the stent in position on its delivery device. Such devices have the advantage that their external surface is smooth, which minimizes trauma to the bronchial walls. Examples of stents using this type of delivery system include the Niti-S, Micro-Tech, and Alveolus AERO (see Fig. 28.1). In these stents, deployment is achieved by the withdrawal of the outer catheter, while the delivery device is held stationary by its proximal end.
Covered SEMAS vary in the proportion of their length that is covered. Ultraflex always have at least 0.5 cm uncovered at each end, while Niti-S and Alveolus are entirely covered. Although in principle these distinctions may appear relevant if, for example, it is important to leave uncovered a lobar take-off, or when tumor ingrowth threatens the entire length of a main bronchus, in practice it is rare for the length of the covering to affect the choice of stent in the author’s experience.
A Few Words Concerning the Literature and Terminology
At the time of writing, there have been no randomized controlled trials assessing the insertion methods, or clinical effectiveness, of metallic airway stents. The literature therefore comprises case series, expert reviews and consensus statements from groups of leading practitioners. Aspects of the practice of metallic airway stenting vary widely between practitioners and centers, and the lack of an established evidence base means that any advice offered about stent choice, patient selection, or deployment techniques will necessarily strike some readers as different or even wrong. I have tried, wherever possible, both to reflect the breadth of published practice but at the same time to keep discussion of different options appropriate and practical.
For the sake of brevity, I shall use terms such as “malignant central airway obstruction” to mean obstruction of the central airways caused by malignant disease, and “benign aerodigestive fistula” to mean one caused by benign disease. This shorthand is not intended to imply that such a fistula, for example, is benign in its effect upon the patient.
Indications for Metallic Stent Insertion
The principal indications for metallic stent insertion are malignant central airway obstruction and management of malignant aerodigestive fistulae. Metallic stents are also used by some practitioners for the management of airway anastomotic complications following lung transplantation.
Management of Malignant Central Airway Obstruction
Metallic stents have been used in the management of all three types of malignant airway lesion: extrinsic, intrinsic, and mixed. Some authors favor covered metallic stents over uncovered whenever malignant central airway obstruction is the indication, even when the lesion is purely extrinsic, arguing that there is little downside risk to this approach and that a cancer cannot be relied upon not to invade the airway at a later date. A covered stent, however, cannot be deployed across a patent airway side branch without the risk of causing post-obstructive pneumonitis. Choosing an uncovered stent in a situation where there is purely extrinsic compression of the target airway, for example, the right mainstem bronchus, but a patent side branch (say the right upper lobe in this example), minimizes the risk of occluding the side-branch airway. In the author’s practice, the decision over whether to use a covered or uncovered stent is influenced by presence and patency of any airway side branches and the availability or otherwise of any effective anticancer treatment which may limit the risk of subsequent tumor ingrowth.
Some practitioners place a covered stent after debridement of intrinsic airway tumor, with the intention of maintaining airway patency by blocking tumor ingrowth. Others, of whom the author is one, prefer to use debulking therapies for intrinsic tumor, repeated as necessary and complemented by anticancer therapy where possible. This strategy avoids stent complications, which are described later, and in many cases it can be pursued for the remainder of the patient’s life without requiring stent placement.
Malignant Aerodigestive Fistula
Fistulae between the esophagus and the trachea or main bronchi can complicate a number of thoracic malignancies, particularly esophageal carcinoma. Fistulae cause cough, fetor, and recurrent aspiration pneumonia, and survival is frequently very short. The goal of intervention is to seal the airway defect as completely as possible, to prevent further aspiration. Covered airway stents, esophageal stents, or both can be employed. Where the airway defect is situated close to the main carina, either in the distal trachea or the proximal portion of either main bronchus, a covered straight stent is rarely suitable, whether deployed in trachea or main bronchus, because the end of the stent will lie too close to the airway defect and tend to migrate into it. In this situation, a covered Y-stent is ideal. Carinal Y-stents are covered in detail in Chap. 30. Airway-esophageal fistulae are the subject of Chap. 41.
Posttransplant Anastamotic Complications
Metallic airway stenting has been employed to promote healing of anastomotic dehiscence following lung transplantation. Mughal and colleagues described 7 patients treated in this manner at the Cleveland Clinic, all of whom had life-threatening grade 3–4 bronchial dehiscence. A satisfactory final outcome was achieved in 6 of 7 patients, but only 3 of the 7 stents could be removed permanently, even though the authors’ intention had been to place them temporarily. The management of disorders encountered after lung transplantation is covered in Chap. 45.
Benign Central Airway Obstruction
The temporary insertion of some types of metallic stent for the management of airway stenoses due to benign conditions is a controversial practice. In 2005, the Food and Drug Administration in the USA issued a medical device safety alert, warning against metallic tracheal stent use in this context, except where careful consideration had been given to alternative treatment options, including airway surgery and silicone stent insertion. The FDA further recommended avoidance of temporary deployment of metallic stents as a bridging therapy because subsequent removal can lead to serious complications. Perhaps as a consequence of this alert, there was a temporary, marked reduction in referrals for metallic stent removal at a major US interventional bronchoscopy center during 2006–2007. Alarmingly, however, such referrals had risen by 2008 to rates greater than those observed prior to 2005.
Patient Selection and Preparation for Stent Insertion
A successful and appropriate metallic stent insertion depends upon the stent being the right treatment both for the lesion and for the patient. The bronchoscopist should consider the following questions (adapted from Lee, Kupeli and Mehta 2010):
1.
Is a stent the right treatment for this lesion? In particular, are there preferable alternative treatments (surgery, radiotherapy, systemic or debulking treatments for malignant disease; surgery, repeated dilatation, topical treatments for benign stenosis)?
2.
Are the patient’s condition and prognosis suitable both for the procedure and for meaningful palliation to be achieved?
3.
What sort of stent, and of what length, diameter, and configuration, is required?
4.
Is this the right hospital, team, and individual to insert it, or should I refer to a center with different expertise?
5.
Does a stent need to be ordered for this procedure or is it in stock? It is advisable to have more than one stent available of the required dimensions in case the first one is mis-deployed or requires immediate removal for any reason.
Table 28.1 addresses the relative suitability of metallic airway stents in different situations. An example of an ideal patient for metallic stenting would be of good performance status but suffering from breathlessness on exertion, with inoperable non-small cell lung cancer that has relapsed after treatment (say) with chemotherapy and radiotherapy. The patient would have a short, 80% stenosis of the midportion of the left main bronchus, just passable with a 5-mm flexible bronchoscope, and patent distal airways. Such a patient would have symptoms likely to benefit from stenting and a paucity of effective alternative treatments. She would be fit for the procedure and have a favorable lesion with no complicating factors, such as a lobar take-off within the treatment segment. No book can describe precisely what treatment choice to make in any possible situation, but in general decisions regarding stent insertion are influenced by the balance of favorable vs. adverse elements concerning the lesion and the patient, and the presence or absence of effective, alternative treatment options. The patient’s life expectancy should neither be too short (futility, lack of meaningful palliation) nor too long (risk of fracture). Above all, stenting is a palliative procedure, and so the bronchoscopist should never have to persuade the patient to have a stent inserted. This last consideration is particularly important because some patients report few symptoms, even in the presence of apparently severe airway lesions. The temptation for the bronchoscopist is to intervene, especially if the airway lesion appears readily treatable. Caution is advised in such circumstances, however, because no risk is greater than the one that did not need to be taken.
Table 28.1
Patient suitability for metallic airway stenting
Best | Intermediate | Worst | |
|---|---|---|---|
Lesion | Purely extrinsic | Mixed | Purely intrinsic |
Underlying disease | Malignant, poorly responsive to chemo/RT or no further treatment available | Malignant, responsive to chemo/RT | Benign |
Rapidly progressive, metastatic, or untreatable carcinoma (e.g., anaplastic thyroid carcinoma, small cell lung carcinoma not suitable for chemo/RT) | |||
Site of lesion | Mid-trachea | RMB, RBI | Lobar |
Mid-LMB | Main carina | Distal LMB | |
Distal RBI | |||
Lobar take-off in stenosed segment | None | Present but occluded | Present and patent |
Shape of stenosis | Dumbbell | Cylindrical | Conical |
Length of stenosis | Short | Long, extending to proximal or distal airway branch | Long, involving distal airway branches |
Severity of stenosis | 50–95% | <50% or 95–99% | Total occlusion |
Minimal stenosis (e.g., post-ablation) | |||
Distal airways | Patent on CT and endoscopically | Patent on CT, or filled with low-attenuation material | Not identifiable |
Pulmonary artery branch accompanying airway | Patent | Narrowed | Occluded |
Patient condition | Stable, breathless on exertion, ECOG 1–2 | Stable but not breathless | Life-threatening CAO (very suitable but challenging) |
Significant cardiorespiratory instability | |||
Estimated life expectancy | 3–24 months | 1–3 months | < 1 month |
24–36 months | > 3 years |
Pre-procedure Patient Assessment
The work-up for metallic stenting necessarily depends upon the patient’s condition. In emergent near-complete tracheal obstruction, intubation and immediate rigid bronchoscopy by the most experienced available practitioner without any pre-procedure work-up may be entirely appropriate. Almost always, however, in addition to a history and physical examination and standard pre-bronchoscopy tests, it is appropriate to request airway CT scanning, spirometry, a flow-volume loop, and an assessment of breathlessness, such as the MRC or Borg dyspnea scores. Some operators favor a prior flexible bronchoscopy to assess the lesion anatomy. Once these tests have been completed, it will often be possible to arrive at a complete specification for the desired stent. Airway CT scanning, particularly if carried out on a high-speed multidetector scanner, can be used to create multiplanar reformat and virtual bronchoscopy images. These enable the diameter of the stenosed segment and, if present, the adjacent normal airway to be estimated. Where the target bronchus has no segment of normal caliber throughout its length, estimates of its usual caliber can be obtained by measuring the corresponding contralateral airway. Images displayed on lung window settings provide a more accurate impression of the likely endobronchial appearances. Dynamic CT images, obtained during expiration, can further define regions where dynamic airway collapse occurs, sometimes as a consequence of the destruction of airway cartilage by underlying tumor. Standard flexible bronchoscopy provides visual assessment of the severity and length of airway stenoses, the patency of any adjacent lobar take-off (particularly the right upper lobe), and the presence or absence of endobronchial tumor. In addition, the relationship of the stenosed segment to distal or proximal airway branches can be precisely assessed. In lower-volume centers, performing a flexible bronchoscopy prior to stenting permits a stent of the required size to be ordered for the procedure, thereby minimizing the requirement to keep an extensive stock of stent sizes. For many straightforward cases, CT scanning, lung function, and clinical assessment will be sufficient to enable satisfactory treatment planning, with an on-table flexible bronchoscopy providing additional information and enabling final stent selection and sizing. This approach has the benefit of simplicity and economy but relies upon the availability of a full range of stent sizes and types, since the final selection is made during the procedure.
Miyazawa and colleagues assessed the impact of a more sophisticated pre-procedure assessment in patients with lung cancer requiring airway stenting. Dynamic airway CT scanning, flow-volume loops, endobronchial ultrasound (EBUS) and ultrathin flexible bronchoscopy were performed before and after stenting in 64 patients. These techniques were combined to allow the identification of specific choke points (wave-speed flow-limiting segments) within the airways. The CT scans were performed at end-expiration, with 3D airway reformats. Characteristic flow-volume loop appearances could be identified for lesions in three locations: the trachea, around the main carina, and confined to the main bronchi, with more complex stenoses exhibiting a combination of appearances. EBUS was used to assess the integrity of underlying airway cartilage. In some patients, this was found to have been destroyed by extrinsic tumor over a length of airway wall greater than that affected by the stenosis itself. EBUS allowed the authors to select longer stents in this situation, to prevent dynamic airway collapse of the damaged airway. Ultrathin bronchoscopy was employed to identify choke points directly. In this study, a combination of silicone and metallic stenting was employed. Using these pre-procedure assessments, patients with tracheal (n = 20), carinal (n = 16), and bronchial lesions (n = 18) were satisfactorily treated with a single stent procedure alone, with significant improvements in lung function parameters and WHO dyspnea grade. In patients with complex stenoses affecting more than one region of the tracheobronchial tree, however, migration of the choke point after an initial stent procedure was observed. In this group of patients, even though significant improvements in lung function and dyspnea scores were observed after a single procedure, there was a further incremental and statistically significant improvement in each of these parameters after a second stent procedure, in which stent deployment was targeted at the migrated choke points identified by the detailed assessment described. The results of this study are worth emphasizing, because many bronchoscopists might have congratulated themselves after achieving the more modest patient benefits demonstrated after the first stent procedure in these complex lesions. This study provides evidence in favor of the proposition that all such patients should be treated in centers with access to a full range of advanced bronchoscopic techniques and that there is a lot more to successful stenting than knowing how to deploy the stent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


