Contrast-induced acute kidney injury (CIAKI) is a leading cause of hospital-acquired acute kidney injury, and pretreatment with hydroxymethylglutaryl CoA reductase inhibitors (statins) have shown promise in prevention. A systematic review and meta-analysis was performed including randomized controlled trials of short-term high-dose statins (compared with either low-dose statin or placebo) for CIAKI prevention in patients undergoing coronary angiography. Study-specific odds ratios (ORs) were calculated, and between-study heterogeneity was assessed using the I 2 statistic. We used a random-effects model meta-analysis to pool the OR. Twelve RCTs, including 5,564 patients, were included. CIAKI occurred in 94 of 2,769 patients (3.4%) pretreated with high-dose statins and 213 of 2,795 patients (7.6%) in the low-dose or no-statin group (OR 0.43, 95% confidence interval [CI] 0.33 to 0.55, I 2 = 19%, p <0.001). Subgroup analysis showed that the occurrence of CIAKI did not differ in patients with diabetes (OR 0.60, 95% CI 0.43 to 0.85, I 2 = 0%, p = 0.004) or in patients with documented renal insufficiency (creatinine clearance <60 ml/min/m 2 ; OR 0.66, 95% CI 0.45 to 0.96, I 2 = 0%, p = 0.03). In conclusion, pretreatment with high-dose statins, compared with low-dose statins or placebo, in patients undergoing coronary angiography reduces the incidence of CIAKI. This benefit was seen irrespective of the presence of diabetes and chronic kidney disease. Future studies should identify optimum dosing protocols for each statin.
Many previous studies on the use of statins for prevention of contrast-induced acute kidney injury (CIAKI) have been performed. Several meta-analyses of these studies testing lipophilic statins (simvastatin and atorvastatin) have been inconclusive. Since then, large randomized trials have studied this subject using a previously untested statin (rosuvastatin). Our aim was to systematically review literature on the role of statins in prevention of CIAKI, applying pooled data results to study differences in renoprotective effect of different lipophilic and hydrophilic statins and different dosing regimens, including studying subgroups of patients at risk (patients with diabetes mellitus [DM] and patients with chronic kidney disease [CKD]).
Methods
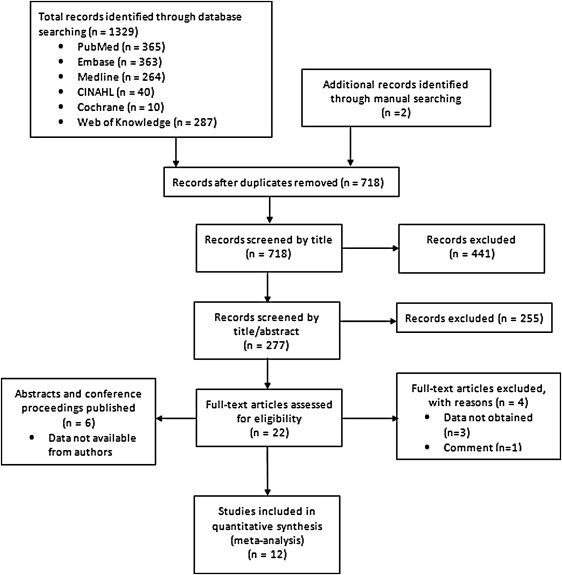
A systematic electronic search of PubMed, EMBASE, Medline, CINAHL, Cochrane Central Register of Controlled trials, and Web of Knowledge for randomized controlled trials (RCTs) of high-dose statins in the prevention of CIAKI from inception to January 2014 was performed. Two independent investigators (AU and MM) reviewed the retrieved database results to determine potential eligibility using the predefined inclusion and exclusion criteria. Disagreements were resolved by a third author (PK). We developed 3 comprehensive search categories and included text words, MeSH terms, and Emtree entry terms, which were combined using the Boolean operator “AND.” The various themes used were contrast medium, renal failure, and statins ( Supplementary File 1 ). Search was limited to human subjects, and no language restriction was used. Bibliographies of the reviewed articles were further manually searched to identify additional reports. Care was taken to avoid duplication. To conduct this meta-analysis, the PRISMA statement for reporting systematic reviews as recommended by the Cochrane Collaboration was followed ( Figure 1 ). Baseline demographics and procedural characteristics of the identified studies ( Tables 1 and 2 ) included mean age, presence of DM, baseline creatinine, type of coronary intervention, contrast type and volume, statin dose and protocol, periprocedural hydration, use of other renoprotective agents, and incidence of CIAKI. The quality of included studies was independently evaluated by 2 reviewers (LJ and AAD) using the guidelines provided by the Cochrane Collaboration tool for assessing risk of bias. Any discrepancy was resolved by a third author (AU). All the studies were assessed for random sequence generation, allocation concealment, blinding of participants, incomplete outcome data selective outcome reporting, and presence of other biases. Each domain of risk was assigned as “yes” for low risk, “no” for high risk, and “unclear” in cases not mentioned ( Supplementary Figure 1 ).
| First Author (Year) | Contrast Type | Average Contrast Volume (ml) | Mean Age (Years) | Diabetics (%) | Mean Baseline SCr (mg/dl) | Definition of CIN | Events (n) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | |||
| Jo (2008) | Iodixanol | 173 | 191 | 65 | 66 | 28.2% | 23.6% | 1.28 | 1.25 | Increase of SCr >0.5 mg/dl or >25% within 48 h | 3 | 4 |
| Toso (2009) | Iodixanol | 151 | 164 | 75 | 76 | 20% | 22% | 1.20 | 1.18 | Increase of SCr >0.5 mg/dl within 5 d | 15 | 16 |
| Xinwei (2009) | Iodixanol, iohexol | 240 | 227 | 65 | 66 | 20% | 22% | 0.82 | 0.83 | Increase of SCr >0.5 mg/dl or >25% within 48 h | 6 | 18 |
| Xia (2009) | Iopamidol | 119 | 113 | 60 | 61 | 22% | 18% | 1.04 | 1.08 | Increase of SCr >0.5 mg/dl or >25% within 72 h | 0 | 3 |
| Acikel (2010) | Iohexol | 105 | 103 | 59 | 61 | 24% | 25% | 0.84 | 0.85 | Increase of SCr >0.5 mg/dl within 48 h | 0 | 1 |
| Ozhan (2010) | Iopamidol | 93 | 97 | 54 | 55 | 15% | 17% | 77.8 | 77.80 | Increase of SCr >0.5 mg/dl or >25% within 48 h | 2 | 7 |
| Patti (2011) | Iobitridol | 209 | 213 | 65 | 66 | 30% | 25% | 1.04 | 1.04 | Increase of SCr >0.5 mg/dl or >25% within 48 h | 6 | 16 |
| Lin (2010) | Iopamidol | 89 | 88 | 62 | 63 | 5% | 7% | 1.56 | 1.50 | Increase of SCr >0.5 mg/dl or >25% within 48 h | 0 | 2 |
| Li (2012) | Ultravist (NI) | 100 | 104 | 66 | 65 | 27% | 29% | 1.48 | 1.49 | Increase of SCr >0.5 mg/dl or >25% within 72 h | 2 | 13 |
| Quintavalle (2012) | Iodixanol (NI, IS) | 177 | 184 | 70 | 70 | 44% | 39% | Increase in cystatin C concentration 10% above the baseline value at 24 hrs post procedure, increase of SCr >0.5 mg/dl or >25% within 48 h | 9 | 37 | ||
| Han (2013) | Iodixanol (NI, IS) | 120* | 110* | 61 | 61 | 100% | 100% | 1.33 | 1.34 | Increase of SCr >0.5 mg/dl or >25% within 72 h | 34 | 58 |
| Leoncini (2013) | Iodixanol | 183 | 172 | 66 | 66 | 20% | 23% | 70 | 69 | Increase in SCr >0.5 mg/dl or >25% within 72 h | 9 | 20 |
| Author, Year | Patients (n) | Inclusion Criteria | NAC (Intervention Group) | Statin Protocol (Intervention Group) | ||||
|---|---|---|---|---|---|---|---|---|
| Statin | Control | Hydration Procedure | Type | Dosage | Duration of Therapy | |||
| Jo (2008) | 113 | 115 | CAG.SCr ≥1.1 mg/dl or CrCl ≤60 ml/min | No | IS, 1 mg/kg/h 12 h before and 12 h after | Simvastatin | 40 mg Every 12 hours | 1 d Pre-procedure and 1 d post-procedure |
| Toso (2009) | 152 | 152 | CAG and/or PCI. CrCl <60 ml/min | 1200 mg Bid 1d before & 1 d after | NS, 1 ml/kg/h 12 h before and after | Atorvastatin | 80 mg/d | 2 d Pre-procedure and 2 d post procedure |
| Xinwei (2009) | 113 | 115 | PCI for ACS | No | NS, 1 ml/kg/h for 6-12 h before and 12 h after | Simvastatin | 80 mg/d, 20 mg/d | 80 mg/d from admission to 1 d before, 20 mg/d after the procedure |
| Xia (2009) | 50 | 50 | CAG or PCI | No | NS, 1000 ml 12 h before and 12 h after | Atorvastatin | 80 mg/d, 10 mg/d | 80 mg/d Pre-procedure for 1 d, 10 mg/d for 6 d after procedure |
| Acikel (2010) | 80 | 80 | CAG. eGFR >60 ml/min per 1.73 m 2 | No | IS, 1 mg/kg/h 4 h before and 24 h after | Atorvastatin | 40 mg/d | 3 d Pre-procedure and 2 d post procedure |
| Ozhan (2010) | 60 | 70 | CAG.SCr ≤1.5 mg/dl or eGFR ≥70 ml/min per 1.73 m 2 | 600 mg Bid pre-procedure | NS, 1000 ml 6 h after | Atorvastatin | 80 mg/d | 1 d Pre-procedure and 2 d post-procedure |
| Patti (2011) | 120 | 121 | CAG and/or PCI. Scr ≤3 mg/dl | No | If CrCl <60 ml/min, 1 ml/kg/h 12 h before to 24 h after | Atorvastatin | 80 mg/d, 40 mg/d | 80 mg 12 h before + 40 mg 2 h before, 40 mg 2 d after the procedure |
| J Lin, 2010 | 46 | 46 | SCr ≥1.1 mg/dl or CrCl ≤60 ml/min | No | N/A | Atorvastatin | 40 mg/d | N/A |
| Li (2012) | 78 | 83 | Acute STEMI | No | IS 1 ml/kg/h before and 12 h after | Atorvastatin | 80 mg/d, 40 mg/d | Given and immediately transferred to catheterization lab (door-to-balloon time ≤1.5 h) followed by long-term atorvastatin 40 mg/d |
| Quintavalle (2012) | 202 | 208 | CKD | 1200 mg Bid 1 d before and same day | NAHCO 3 (in dextrose and water)-3 ml/kg/h 1 h before f/b 1 ml/kg/h during contrast and 6 h after | Atorvastatin | 80 mg/d | Within 24 h before contrast |
| Han (2013) | 1498 | 1500 | Concomitant DM 2 and CKD stage 2 or 3 | No | IS, 1 ml/kg/h 12 h before to 24 h after | Rosuvastatin | 10 mg/d | 2 d Before to 3 d after contrast |
| Leoncini (2013) | 252 | 252 | NSTEMI for early, SCr <3 mg/dl | No | IS, 1 mg/kg/h for 12 h before and 12 h after | Rosuvastatin | 40 mg/d, 20 mg/d | On the day of procedure, and the day after |
Eligible studies were RCTs that evaluated the effect of preprocedural statin therapy on CIAKI in patients undergoing coronary angiography. Inclusion criteria for this meta-analysis were (1) RCTs of human subjects, independent of renal function or other co-morbidities; (2) interventions of interest were high-dose statins, defined by the included studies as atorvastatin 40 or 80 mg, simvastatin 40 or 80 mg, rosuvastatin 10 or 40 mg, or an increase in statin dose from a baseline; (3) control interventions consisted of placebo or lower dose statin than the intervention arm; and (4) equal use of other renoprotective strategies such as saline solution, bicarbonate, and N-acetyl cysteine (NAC) in both intervention and control arms. Reports excluded from the analysis included editorials, narrative reviews, non–peer-reviewed abstracts, and studies evaluating chronic statin use without periprocedural dose alterations.
The primary end point of interest was development of CIAKI. CIAKI was defined as a 25% or 0.5 mg/dl increase in creatinine from baseline, or an increase in cystatin C >10%, within 48 to 120 hours of intravenous contrast exposure, as per standard accepted definitions in literature.
All outcome comparisons and treatment effects were calculated with RevMan, version 5.2 (Cochrane Collaboration, Oxford, United Kingdom). The summary odds ratio (OR) and 95% confidence intervals (CIs) were estimated using a fixed-effects method if I 2 was 0%. To control for heterogeneity if present, random-effect models were used for this meta-analysis as their assumption accounts for presence of variability among the studies. We calculated the I 2 statistic to evaluate the percentage of heterogeneity among the trials. When interpreting heterogeneity, I 2 values <25% were considered as low heterogeneity, 25% to 50% as moderate, and >75% as high. A p value of <0.05 was used as the level of significance. The results are reported in a forest plot with 95% CIs. ORs were calculated for each outcome. Potential publication bias was assessed using Begg funnel plots ( Supplementary Figure 2 ) of natural log of OR and standard error. The Cochrane calculator was used to determine the number needed to treat to prevent 1 incidence of CIAKI.
We performed several subgroup analyses to determine the efficacy of each individual statin (simvastatin, atorvastatin, and rosuvastatin) and also grouping statins according to the statins’ lipophilicity (simvastatin and atorvastatin) and hydrophilicity (rosuvastatin). Analysis of the data in high-risk patient subgroups with DM and CKD was performed. We also grouped the studies based on whether the control arm received statin or placebo. Further subgroup analysis was based on coadministration of NAC and the continent of origin of the included study populations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


