Although previous studies have shown that frequent ventricular premature complexes (VPCs) in patients with established heart disease are associated with increased risk of cardiac mortality, the significance of VPCs in general populations is unclear. The aim of this study was to assess the association between VPCs and risk of sudden cardiac death or total cardiac death in general populations by conducting a meta-analysis of published research. The electronic databases MEDLINE and Embase were searched for relevant studies. Data were abstracted using standardized forms. Study-specific relative risk estimates were pooled using a random-effects meta-analysis model. Eleven studies comprising a total of 106,195 participants sampled from general populations were included. Studies generally defined frequent VPCs as occurring ≥1 time during a standard electrocardiographic recording or ≥30 times over a 1-hour recording. The prevalence of frequent VPCs in the studies ranged from 1.2% to 10.7%. The overall adjusted relative risk for sudden cardiac death comparing participants with frequent VPCs versus those without frequent VPCs was 2.64 (95% confidence interval 1.93 to 3.63). The corresponding value for total cardiac death was 2.07 (95% confidence interval 1.71 to 2.50). Although most studies made attempts to exclude high-risk subjects, such as those with histories of cardiovascular disease, they did not test participants for underlying structural heart disease. In conclusion, findings from observational studies in general populations indicate that frequent VPCs are associated with a substantial increase in the risk for sudden cardiac death and total cardiac death. Further study is needed to determine the role of confounding and underlying structural heart disease in the observed association and its utility in cardiovascular risk prediction.
Ventricular premature complexes (VPCs) are common findings on electrocardiography in healthy subjects. Observational studies have documented VPCs in about 6% of the general population. The significance of VPCs in subjects with existing coronary heart disease is relatively well understood; for instance, it has been shown that the presence of recurrent VPCs in survivors of myocardial infarction is an indicator of poor prognosis. VPCs may trigger fatal cardiac arrhythmias in patients with underlying heart disease. However, the role of VPCs in generally healthy subjects remains controversial. Whether frequent VPCs are associated with sudden cardiac death (SCD) or total cardiac death (TCD) in subjects without underlying heart disease is not clear, although VPCs are commonly believed to be less harmful in this setting. The objective of the present study was to provide a systematic review and meta-analysis of the available evidence on the association between VPCs and the risk for SCD or TCD in general populations.
Methods
Two investigators (FA, SE) conducted the primary search in PubMed through October 2012, using free-text or Medical Subject Headings terms related to the exposure (i.e., “ventricular premature complexes,” “premature ventricular contractions,” and “premature ventricular complexes”) and those related to the outcome (i.e., “sudden cardiac death,” “sudden death,” “death,” and “mortality”) without restriction to publication date. Supplementary searches were also conducted in the Web of Knowledge and Embase using the same search terms. We supplemented the electronic searches by manually scanning the reference lists of relevant reports.
The PubMed search retrieved 928 citations, from which 95 potentially relevant reports were selected for further review on the basis of the titles and abstracts. After reviewing the full-text reports, 9 studies that met the prespecified inclusion criteria were identified. In addition, 2 distinct eligible studies were identified from the Web of Knowledge and Embase search and scanning of reference lists of the retrieved reports, yielding a total of 11 studies included in the present review ( Figure 1 ).
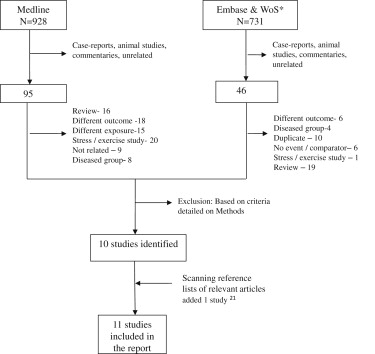
Prospective cohort, nested case-control, and retrospective cohort studies based in general populations that assessed the association of VPCs with TCD or SCD outcomes were eligible for this review. We excluded case-control and cross-sectional studies to minimize bias. Other exclusions were studies (1) that selected participants on the basis of specific characteristics (e.g., those with coronary heart disease at baseline) or were conducted in hospitalized patients, (2) that assessed outcomes other than SCD or TCD, and (3) that measured stress or exercise-induced VPCs.
SCD is untimely cardiac-related death that occurs in an otherwise asymptomatic subject without warning symptoms. The exact definition of “sudden” or “untimely” has been variable across studies, with the major difference being the length of time window between the onset of cardiac symptoms and death, which could span anywhere from <1 to 24 hours. TCD is death in which cardiac disease is confirmed as the primary cause. Coronary heart disease contributes to most cardiac mortality and a substantial proportion is sudden in nature.
Studies generally defined frequent VPCs as occurring ≥1 time during a 10-second or 2-minute electrocardiographic (ECG) recording or ≥30 times over a 1-hour recording. If a study used multiple criteria to define frequent VPCs, we considered the definition that was most consistent with the rest of the studies. For example, Abdalla et al defined frequent VPCs on a 2-minute ECG recording as “at least one VPC” and also as “more than one VPC,” and we used the former definition for the meta-analysis.
Two investigators (FA, SE) extracted the relevant information using a standardized abstraction form. The abstracted information included general characteristics of the studies ( Table 1 ); definition of exposure, outcome, and relevant methodologic details ( Table 2 ); and estimate of association between frequent VPCs and either SCD or TCD (along with their 95% confidence intervals [CIs] and p values) and the covariates adjusted for in regression models ( Table 3 ).
| Study | Study Name | Location | Study Design | Total n | Mean Age (or Range) (yrs) | Male Patients | VPC Prevalence | Average Follow-Up (yrs) | SCD (n) | TCD (n) | NOS Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdalla et al (1987) | MRFIT (ancillary ∗ ) | United States | PC | 15,637 | 35–57 | 100% | 4.4% | 7.5 | 41 | 131 | 8 |
| Sajadieh et al (2006) | Copenhagen Holter study | Denmark | PC | 678 | 65 | 59% | 8% | 5 | NA | 66 | 8 |
| Engel et al (2007) | NA | United States | PC | 43,671 | 56 | 90% | 3.8% | 5.5 | NA | 3,768 | 6 |
| Fisher and Tyroler (1973) | NA | United States | RC | 1,214 | 35–69 | 100% | 6.7% | 11 | 59 | NA | 6 |
| Cheriyath et al (2011) | ARIC | United States | PC | 14,574 | 53 | 43% | 4.8% | 14 | 130 | 288 | 9 |
| Hirose et al (2010) | JMS cohort study | Japan | PC | 11,158 | 59 | 39% | 1.2% | 11.9 | NA | 92 | 8 |
| Cullen et al (1982) | Busselton study | Australia | PC | 2,119 | 40–79 | 50% | 4.1% | 13 | NA | 15 | 8 |
| Rabkin (1984) | Manitoba study | Canada | PC | 3,983 | 31 | 100% | 10.7% | 30 | 70 | NA | 7 |
| Stein et al (2010) | CHS | United States | NCC | 1,814 | >65 | 43% | 5.3% | 13 | 49 | NA | 6 |
| Chiang et al (1969) | TCHS | United States | PC | 5,129 | >16 | NR | 3.6% | 6 | 45 | NA | 7 |
| Bikkina et al (1992) | FHS | United States | PC | 6,218 | 54 | 45% | 7% | 6 | NA | 163 | 9 |
| Study | Population/Sampling Method | Participant Exclusion Criteria | Exposure Definition | Exposure Measurement Method | Outcome Definition | Outcome Ascertainment | Variables Included in Maximally Adjusted Model |
|---|---|---|---|---|---|---|---|
| Abdalla et al (1987) | Ancillary project of first screening examination for MRFIT | Hx of MI, DM, other abnormalities on ECG | Presence of any VPC on 2-minute ECG | 2-minute ECG | SCD || | Social Security data, NOK interview, hospital record review | Age, BP, total cholesterol, smoking status |
| Sajadieh et al (2006) | Copenhagen Holter study, apparently healthy middle and elderly patients, random ∗ | Hx of heart disease and stroke | >30 VPCs/h | 48-h ambulatory ECG (Holter) | Total CVD death | National Central Patient Registry | BP, DM, physical exercise |
| Engel et al (2007) | Veterans at Palo Alto VA Medical Center who underwent ECG for any reason | ECG of AF and paced rhythms | Presence of any VPCs on 12-lead ECG | 10-second ECG | Total CVD death and AMI | Social Security Death Index, California Health Department Service and VA database | Age, BMI, findings on ECG |
| Fisher and Tyroler (1973) | White male factory workers in Canton, North Carolina | None | Presence of any VPCs on 12-lead ECG | 10-second ECG | SCD, ¶ , # total CHD death | Death certificate | Age |
| Cheriyath et al (2011) | ARIC, population-based sample, complete | Hx of CHD or stroke | Presence of any VPCs on 2-minute ECG | 2-minute ECG | SCD ¶ total CHD death | Hospital record, death certificate, questionnaire and interview of NOK | Age, race, gender, education, smoking status, BMI, serum K, Mg, LDL-C/HDL-C, DM, HTN, HR, anti-HTN, antiarrhythmic |
| Hirose et al (2010) | JMS cohort study, study districts in rural Japan, complete | Hx of MI or stroke | Presence of any VPCs on 12-lead ECG | 10-second ECG | TCD | Death certificate from local public centers | BMI, SBP, TC, HDL-C, blood glucose |
| Cullen et al (1982) | Busselton study, unselected subjects | Separate analysis by angina status | Presence of any VPCs on 12-lead ECG | 10-second ECG | TCD †† | Registrar of death | Age and gender |
| Rabkin (1984) | Manitoba study, men who were either pilots or pilots in training in the Royal Canadian Air Force in World War II, complete † | Previous MI, angina | Presence of any VPCs on 12-lead ECG | 10-second ECG | SCD # | Annual letters, medical records | Age |
| Stein et al (2010) | CHS, population based, random, ‡ patients who experienced SCD during follow-up and controls § | Hospitalized or nursing home patients | >30 VPCs/h | 24-h ambulatory ECG (Holter) | SCD ∗∗ | Cardiologists’ record review | Age, gender, MI history, DM medication, β blocker |
| Chiang et al (1969) | TCHS, complete, community of Tecumseh | None | Presence of any VPCs on 12-lead ECG | 10-second ECG | SCD ¶ | Death certificate | Age |
| Bikkina et al (1992) | FHS, surviving patients and offspring of the original cohort members | Separate analysis by CHD status | >30 VPCs h or complex VPCs | 48-h ambulatory ECG (Holter) | Total CHD death and AMI | Medical record review, hospital record and pathology report | Age, TC, HDL-C, BMI, HTN, smoking, SBP, DM, CHF, anti-HTN, β blocker, antiarrhythmic |
∗ Random sample of 60% subjects with no or 1 self-reported CVD risk factor.
† Pilots who at the time had electrocardiographic records in addition to general medical tests. Eligibility: electrocardiographic abnormality and no evidence of ischemic or valvular heart disease (clinical or on ECG).
‡ Randomly selected government-sponsored health insurance (Medicare) cohort members free of prevalent or incident CVD and free of MRI-detectable infarcts who did not have illness expected to lead to early death.
§ Controls were participants alive at the time of death of the patient who had not died at follow-up; each patient was matched on age within 5 years, gender, β-blocker use, history of MI, and use of oral hypoglycemic medications with 2 controls.
|| Witnessed death occurring <1 hour after onset of acute symptoms.
¶ Death occurring <1 hour after onset of acute signs and symptoms.
# SCD from CHD defined as death occurring <24 hours after onset of acute signs and symptoms.
∗∗ SCD defined as a sudden pulseless condition (presumably, but not definitely, ventricular fibrillation) due to cardiac cause in an otherwise stable patient. Patients who had their events at emergency departments or out of the hospital were included, whereas those already hospitalized or at nursing homes with multiple co-morbidities were excluded.
†† Definition from International Classification of Disease, Eighth Revision, codes 390 to 458, 746, and 747.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


