Chronic kidney disease (CKD) and atrial fibrillation (AF) frequently coexist. However, the extent to which CKD increases the risk of thromboembolism in patients with nonvalvular AF and the benefits of anticoagulation in this group remain unclear. We addressed the role of CKD in the prediction of thromboembolic events and the impact of anticoagulation using a meta-analysis method. Data sources included MEDLINE, EMBASE, and Cochrane (from inception to January 2014). Three independent reviewers selected studies. Descriptive and quantitative information was extracted from each selected study and a random-effects meta-analysis was performed. After screening 962 search results, 19 studies were considered eligible. Among patients with AF, the presence of CKD resulted in an increased risk of thromboembolism (hazard ratio [HR] 1.46, 95% confidence interval [CI] 1.20 to 1.76, p = 0.0001), particularly in case of end-stage CKD (HR 1.83, 95% CI 1.56 to 2.14, p <0.00001). Warfarin decreased the incidence of thromboembolic events in patients with non–end-stage CKD (HR 0.39, 95% CI 0.18 to 0.86, p <0.00001). Recent data on novel oral anticoagulants suggested a higher efficacy of these agents compared with warfarin (HR 0.80, 95% CI 0.66 to 0.96, p = 0.02) and aspirin (HR 0.32, 95% CI 0.19 to 0.55, p <0.0001) in treating non–end-stage CKD. In conclusion, the presence of CKD in patients with AF is associated with an almost 50% increased thromboembolic risk, which can be effectively decreased with appropriate antithrombotic therapy. Further prospective studies are needed to better evaluate the interest of anticoagulation in patients with severe CKD.
Thromboembolic events are one of the most feared complications of atrial fibrillation (AF). Chronic kidney disease (CKD) is relatively prevalent in patients with AF. The extent to which the presence of CKD may increase the risk of thromboembolism in patients with AF has not yet been fully elucidated. Oral anticoagulation is the mainstay of thromboembolic prevention in patients with AF, but data on efficacy and safety in the CKD and dialysis population have been scarce and contradictory. Our aim was to systematically evaluate, through a meta-analysis method, the impact of the presence of CKD in patients with AF as regards risk of thromboembolism and the potential benefit of anticoagulation in that setting.
Methods
We performed a search in MEDLINE (by way of Ovid and PubMed), EMBASE, and Cochrane (from inception to January 3, 2014) databases using the following search string: “atrial fibrillation” AND (“renal failure” OR “chronic renal disease” OR “dialysis”) AND (“stroke” OR “thromboembolism”). The reference lists of the accessed full-text reports were further researched for sources of potential information relevant to this analysis. The authors of full-text reports and abstracts were contacted by e-mail to retrieve additional information.
Only longitudinal studies assessing the occurrence of a composite end point of stroke or systemic embolism (and including transient ischemic attack) during follow-up in patients with AF were considered for inclusion. Both registries and randomized trials were considered eligible for analysis. The methods sections of evaluated studies were reviewed to confirm the suitability and composition of the reported end point. Studies assessing only stroke (either ischemic, hemorrhagic, or a composite of both) and providing no data on systemic embolism were not considered representative of the full spectrum of thromboembolism in AF and were excluded from analysis. Similarly, studies only reporting stroke or systemic embolism in association with myocardial infarction, hospitalization, or death not due to stroke or systemic embolism were not included.
To be included in the systematic review, the studies needed to have a design allowing extraction of information concerning at least 1 of the 2 main aims of this study: (1) assessment of the incidence of stroke and systemic embolism in patients with AF according to the presence of CKD (including dialysis treatment) and (2) estimating the impact of anticoagulation in patients with CKD and AF. The population, intervention, comparison, and outcome approach was used for this aim. The population of interest included patients with nonvalvular AF with CKD or treated with dialysis. The term end-stage CKD was used for patients with disease requiring renal replacement therapy, either dialysis or transplantation. Non–end-stage CKD was used for the remaining patients with renal disease. The intervention was anticoagulation. Comparisons were performed among the following groups: adjusted-dose warfarin (target international normalized ratio of 2 to 3) versus no treatment, aspirin or low dosage non–adjusted-dose warfarin (target international normalized ratio <1.5); warfarin versus novel oral anticoagulants; and novel oral anticoagulants versus aspirin. The outcome has been defined previously.
Two independent reviewers (RP and SCB) screened all abstracts and titles to identify potentially eligible studies. The full text of these potentially eligible studies was then evaluated to determine the eligibility of the study for the review and meta-analysis. Disagreements regarding eligibility were resolved by consensus with the help of a third reviewer (SB).
Data extraction and presentation for the preparation of this report followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses group. The following data were extracted for characterizing each patient sample in the selected studies, whenever available: criteria for defining CKD, number of patients with CKD (and when available, number in each estimated glomerular filtration rate [eGFR] category of the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative classification ) or on dialysis in each study, and type and frequency of antithrombotic treatment (warfarin or other vitamin K antagonists, novel oral anticoagulants, aspirin, or other antiplatelet agents).
Data were pooled using random effects, according to the Mantel-Haenszel model, through Review Manager (RevMan), version 5.1 (The Nordic Cochrane Center, The Cochrane Collaboration, 2011, Copenhagen, Denmark). The measurement of treatment effect and AF, CKD, or dialysis exposure was performed using dichotomous adjusted hazard ratios (HR) and 95% confidence intervals (CI). Pairwise comparisons were performed for the primary end point in the settings defined in the third paragraph of the Methods section. Comparison of the treatment effect of adjusted-dose warfarin versus the novel oral anticoagulants was performed through the use of risk ratios (number of events or the incidence in each treatment group) from randomized controlled trials. Additional sensitivity analyses were performed, whenever data were available, regarding end-stage CKD on dialysis treatment. Statistical heterogeneity on each outcome of interest was assessed and quantified using the Cochran Q test and the I 2 statistic, respectively. The I 2 statistic describes the percentage of total variation across studies due to heterogeneity rather than chance. Values of <25%, 25% to 50%, and >50% are by convention classified low, moderate, and high degrees of heterogeneity, respectively. The presence of publication bias was evaluated through the use of funnel plots if the appropriate requisites concerning the minimum number of included studies in a forest plot were met (at least 10 studies).
Results
Overall, 962 entries were retrieved for title and abstract analysis. Of these, 783 were excluded as they did not meet inclusion criteria for the meta-analysis and 106 were duplicate entries. The remaining 73 studies were carefully evaluated, and after full-text review, only 19 studies (all full-text reports) were finally considered eligible. The stepwise selection process is illustrated in Figure 1 . There was complete agreement between investigators on the inclusion of all the selected trials. Information on risk stratification, study design, number of participants, and the main findings in each study are provided in Table 1 . Following the predefined inclusion and exclusion criteria, ≤5 studies were included in each of the traced forest plots. Accordingly, no funnel plots were drawn.
| Author, Ref | Study Design, Acronym | Sample Size (pts) | Intervention or Baseline Anti-thrombotics | Dialysis pts (%) HD/PD | CKD pts (%) eGFR Cutoff (ml/min) | Length of FUP (yrs) | Association of CKD With Stroke and/or SE | Anticoagulation in pts With CKD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable Outcome | HR 95% CI | Endpoint | Intervention Outcome | HR 95% CI | |||||||
| Roldan | Prospective Single-center Observational | 978 | Acenocoumarol 100% | NA | eGFR 30–59, 28% eGFR <30, 3% | Median 2.4 | eGFR NS | 1.06 0.69–1.63 | Stroke or SE | — | — |
| Banerjee | Retrospective Regional (4 hospitals) Observational | 5,912 | VKA 52.5% Antiplatelet 30.8% None 26.0% | Baseline or FUP 2.2% | eGFR 30–59, 20.2% eGFR <30, 5.8% | Mean 2.5 | Renal impairment NS eGFR NS | 1.06 0.75–1.49 1.09 0.84–1.41 | Stroke or SE Stroke or SE | — | — |
| Olesen | Retrospective Nationwide Observational | 132,372 | W 28.3% A 18.9% W + A 8.4% | Baseline RRT 0.7% FUP RRT 1.0% (78%HD/15% PD) | NA | Maximum 12 | CKD (non-endstage) ↑ RRT ↑ | 1.49 1.38–1.59 1.83 1.57–2.14 | Stroke or SE Stroke or SE | W NS ↓ trend W ↓ | 0.84 0.69–1.01 0.44 0.26–0.76 |
| Friberg | Retrospective Nationwide Observational | 170,291 | W baseline 40% W baseline/FUP 47% | NA | Renal disease ≈6.0% | Mean 1.5 | Renal failure ↑ | 1.16 1.05–1.28 | Ischemic stroke/US/TIA/SE | — | — |
| Eikelboom Connolly | RCT AVERROES | 5,599 | Apixa vs A (1:1) 5mg ∗ bid vs 81–324 mg od | Exclusion criteria | eGFR 30–60, 30.3% eGFR ≤30, 0.4% | Mean 1.1 | Stage III CKD ↑ | 1.6 NA (p <0.01) | Stroke or SE | Apixa vs A ↓ | 0.32 0.18–0.55 |
| Cha | Retrospective Single-center Observational | 695 | W 26.0% A 61.4% None 12.5% | NA | eGFR <60, 20.8% | Median 5.5 | eGFR <60 ↑ | 3.63 1.57–8.42 | Ischemic stroke/TIA/SE | W ↓ | 0.39 0.16–0.99 |
| Patel Piccini | RCT ROCKET-AF | 14,264 | Riva vs W (1:1) 20 mg ∗ od | Exclusion criteria | eGFR <50, 20.7% | Median 1.9 | eGFR ↑ | 1.12 1.07–1.16 | Stroke or SE | Riva vs W NS | 0.88 0.65–1.19 |
| Hart | RCT SPAF-III | 1,936 | Low risk A 46.1% High risk 1:1 W vs low W + A † 53.9% | NA | eGFR 30–59, 41.6% eGFR ≤30, 1.5% | Low risk Mean 2 High risk Mean 1.1 | Stage III CKD (pts treated with A) ↑ | 2.0 1.2–3.3 | Ischemic stroke or SE | W vs A + low W ↓ | 0.24 0.10–0.38 |
| Granger Hohnloser | RCT ARISTOTLE | 18,201 | Apixa vs W (1:1) | Exclusion criteria | eGFR ≤50, 16.6% eGFR ≤30, 1.5% | Median 1.8 | — | — | Stroke or SE | Apixa vs W NS | 0.79 0.55–1.14 |
| Lai | Retrospective Single-center Observational | 399 | W 58.1% A 41.4% | 23% HD | eGFR <60, 100% eGFR <15, 33.1% | Mean W 2.6 No W 1.9 | — | — | Ischemic stroke or SE | W ↓ | 0.28 0.16–0.50 |
| Go Singer | Prospective Multicentric Observational ATRIA | 10,908 | W None | NA eGFR <15 0.9% ♂ 0.7% ♀ | eGFR 45–59, 18.5% eGFR <45, 9.9% | Pt-yrs 33,165 | Proteinuria ↑ eGFR <45 ↑ | 1.54 1.29–1.85 1.39 1.13–1.71 | Ischemic stroke or SE Ischemic stroke or SE | — | — |
| Connolly Hijazi | RCT RE-LY | 17,951 | High Dabi/Low Dabi vs W 150 mg bid 110 mg bid (1:1:1) | Exclusion criteria | eGFR <50, 18.8% | Median 2.0 | — | — | Stroke or SE | High Dabi vs W ↓ Low Dabi vs W NS | 0.56 0.37–0.85 0.85 0.59–1.24 |
∗ Apixa (from 5 mg bid to 2.5 mg bid) and Riva (from 20 mg od to 15 mg od) presented dose adjustment for patients with a certain degree of CKD.
† Target INR in all RCT was 2.0 to 3.0, except in the A + low dose W arm of the SPAF-III trial, where a mean INR of 1.3 was achieved as a result of the daily 1 to 3 mg warfarin alongside with A 325 mg od.
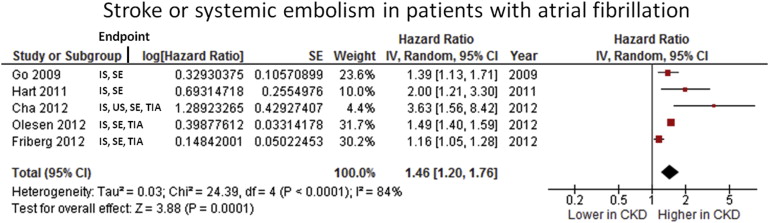
Of the selected studies, 10 provided information concerning the impact of CKD on the incidence of stroke or systemic embolism in patients with AF. Different equations were used for estimating the eGFR and classifying patients as having CKD: Cockcroft-Gault formula was used in 5 studies and the Modification of Diet in Renal Disease was used in 5. Also, in 2 investigations the chronic kidney disease epidemiology collaboration and cystatin C clearance were also used for assessing the safety and efficacy of apixaban and dabigatran at different levels of eGFR. The remaining selected studies for the systematic review did not use any of these, because the diagnosis of CKD was retrieved from codification. A cutoff of 50 to 60 ml/min was used in most studies for defining the presence of CKD.
According to data on Figure 2 , in patients with AF the presence of CKD was associated with a higher rate of thromboembolic events (HR 1.46, 95% CI 1.20 to 1.76, p = 0.0001). All included studies were in favor of the association of CKD with an increase in thromboembolism in patients with AF. However, their high heterogeneity is shown by the I 2 statistic of ≥80%. Information concerning the risk of stroke or systemic embolism in patients with AF who were also on dialysis was provided by only 1 study: in the national Danish registry thromboembolism was found to be increased in this specific population (HR 1.83, 95% CI 1.56 to 2.14, p <0.00001).
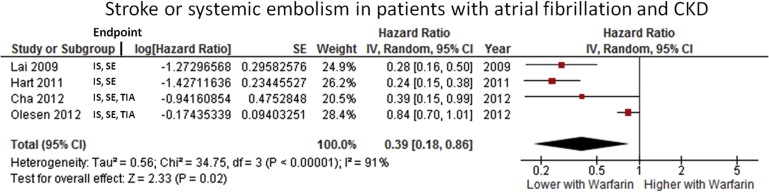
Baseline data, design, and the main findings of trials providing information regarding warfarin in this setting are listed in Table 1 . Information concerning time in therapeutic range is only known for the 3 included randomized controlled trials of the novel oral anticoagulants controlled with warfarin (64% in Randomized Evaluation of Long-Term Anticoagulation Therapy [RE-LY], 55% in Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation [ROCKET-AF], and 62% in Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation [ARISTOTLE] trials). As regards the presence of heparin treatment in patients on dialysis, this information was absent or lacked details concerning the protocol used in the included studies.
The use of warfarin was associated with a major decrease in thromboembolic events (HR 0.39, 95% CI 0.18 to 0.86, p <0.00001) in patients with CKD. The effect was present in all studies but one (which revealed a strong trend for benefit of warfarin; Figure 3 ). Despite the overall favorable trend, a high heterogeneity, I 2 statistic of 91%, was observed driven by the differences in treatment effect. Only 1 study assessing the role of warfarin in the prevention of thromboembolism in patients on dialysis met the inclusion criteria for this meta-analysis. There, warfarin displayed a protective effect (HR 0.44, 95% CI 0.26 to 0.74, p = 0.002). Also, the use of warfarin did not lead to an increased risk of bleeding (HR 1.27, 95% CI 0.91 to 1.77, p = 0.15).