The occurrence of depression in patients with coronary heart disease (CHD) substantially increases the likelihood of a poorer cardiovascular prognosis. Although antidepressants are generally effective in decreasing depression, their use in patients with CHD is controversial. We carried out a meta-analysis to evaluate the health effects of selective serotonin reuptake inhibitors (SSRIs) versus placebo or no antidepressants in patients with CHD and depression. Observational studies and randomized controlled trials (RCTs) were searched in MEDLINE, EMBASE, PsycINFO, Cochrane Controlled Clinical Trial Register and other trial registries, and references of relevant articles. Primary outcomes were readmission for CHD (including myocardial infarction, unstable angina, and stroke) and all-cause mortality; the secondary outcome was severity of depression symptoms. Seven articles on 6 RCTs involving 2,461 participants were included. One study incorrectly randomized participants, and another was a reanalysis of RCT data. These were considered observational and analyzed separately. When only properly randomized trials were considered (n = 734 patients), patients on SSRIs showed no significant differences in mortality (risk ratio 0.39, 95% confidence interval 0.08 to 2.01) or CHD readmission rates (0.74, 0.44 to 1.23) compared to controls. Conversely, when all studies were included, SSRI use was associated with a significant decrease in CHD readmission (0.63, 0.46 to 0.86) and mortality rates (0.56, 0.35 to 0.88). A significantly greater improvement in depression symptoms was always apparent in patients on SSRIs with all selected indicators. In conclusion, in patients with CHD and depression, SSRI medication decreases depression symptoms and may improve CHD prognosis.
Occurrence of depression in patients with coronary heart disease (CHD) has been shown to substantially increase the likelihood of a poorer cardiovascular prognosis. Although antidepressants are generally effective in decreasing depression, their use in patients with CHD is controversial. Some studies have shown an increased CHD risk associated with depression and use of tricyclic antidepressant medication. As a consequence, selective serotonin reuptake inhibitors (SSRIs) currently represent the mainstay of pharmacologic treatment for depressed patients with CHD. However, some studies have supported the efficacy and safety of SSRIs for such patients, whereas other observational analyses have reported an association between SSRI and serious cardiovascular events. Therefore, uncertainty remains on the effects of SSRIs in patients with depression and CHD. We carried out a meta-analysis to summarize evidence on the effects of SSRI versus placebo or no antidepressants in all-cause mortality and readmission for CHD in patients with CHD and depression.
Methods
Studies evaluating the health effects of any SSRI on depressed patients with CHD were retrieved through a search in MEDLINE (PubMed), EMBASE, PsycINFO, CENTRAL, and the Cochrane Controlled Clinical Trial Register ( http://mrw.interscience.wiley.com/cochrane/ ) from inception through April 30, 2010. We did not apply any language restriction. Search terms were “SSRI*” or “selective serotonin reuptake inhibitor*” or “citalopram” or “dapoxetine” or “escitalopram” or “fluoxetine” or “fluvoxamine” or “paroxetine” or “sertraline” or “zimelidine,” “depress*,” “coronary heart disease*” or “heart disease” or “cardiovascular disease*” or “myocardial infarction” or “angina” or “acute coronary syndrome” in all fields. In addition, we searched 2 clinical trial registries, http://apps.who.int/trialsearch/ and http://www.clinicaltrials.gov , and screened bibliographies of related systematic reviews and articles meeting inclusion criteria. To locate unpublished material and avoid systematic (i.e., publication) bias, we searched the Food and Drug Administration or other sources. Case–control and cohort studies and randomized controlled trials (RCTs) were considered for inclusion. Studies without a control group or with active control groups (other antidepressant medications) were excluded. We only considered studies enrolling patients with cardiovascular disease and depression according to Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition or Fourth Edition criteria. Studies enrolling patients with depression and dysthymia were considered if ≥80% of subjects were patients with depression. We excluded other reports including mixed populations unless it was possible to extract data for the subgroup of patients with CHD and depression.
Primary outcomes related to safety: readmission for CHD (including myocardial infarction, unstable angina, and stroke) and all-cause mortality. Secondary outcomes pertained to SSRI efficacy in decreasing depression: mean decrease in depression symptoms from baseline to end of follow-up, response rate (defined as percentage of patients who had ≥50% decrease in their baseline depression score during follow-up), and remission rate (proportion of patients whose depression score decreased below a certain cutoff indicating normality during follow-up). All validated instruments were accepted to evaluate depression symptoms. When a study used >1 depression scale, we applied an a priori determined hierarchy (based on diffusion in the field) and extracted data on the depression scale that was highest on this ordered list: Hamilton Depression 17-item rating scale, Hamilton Depression 24-item rating scale, Beck Depression Inventory, and other validated depression scales.
If results were presented for >1 time point, the last available result was extracted. Data extraction and quality assessment were made independently by 2 reviewers. No attempt was made to blind for authors or institutions. According to the method described by Juni et al, we assessed aspects of the reported methodologic quality of each study pertaining to randomization (generation of allocation sequences and concealment of allocation), blinding, and adequacy of analyses. For observational studies or observational subgroup analyses of randomized trials, we assessed the comparability across groups at baseline for confounding factors and examined whether analyses were adjusted adequately for confounders. We further extracted generic and trade names of an SSRI, type of control used, dosage, frequency, duration of treatment, patient characteristics (gender, average age, definition of major depression, type of coronary artery disease), type of depression scale extracted, study design, study size, duration of follow-up, type and source of financial support, and publication status.
Our main analysis included data from randomized trials only. Dichotomous outcomes for all-cause mortality, readmission for CHD, and response and remission rates were summarized in risk ratios (RRs) and corresponding 95% confidence intervals. We anticipated that >1 scale was adopted to measure depression symptoms in the included studies. Similarly, we expected that studies would differ in their definitions to indicate mean decrease in depression symptoms. We therefore planned to use standardized mean differences to summarize continuous outcomes. When SDs of the mean difference in depression symptoms score from baseline to end of follow-up were not reported and impossible to compute from other data, we conservatively used the largest SDs of the mean score (at baseline or at end of follow-up) of each group. We used random-effects models that account for between-study variance. In sensitivity analyses, we used fixed-effect models to verify the level of agreement between summary estimates from the 2 models. The Mantel–Haenszel approach was used to combine unadjusted results; the generic inverse variance approach when adjusted results also were present.
In secondary analyses, we combined results from randomized trials and observational studies or observational subgroup analyses of randomized trials to explore if data from the combined set supported those derived from randomized trials only. When adjusted estimates were included in meta-analyses, a random-effect generic inverse variance approach was used, computing standard errors from 95% confidence interval according to standard methods. In all comparisons, statistical heterogeneity was quantified using the I 2 statistic, which describes percent variation across studies that is attributable to heterogeneity rather than to chance and the corresponding p value from chi-square test. I 2 values of 25%, 50%, and 75% are typically interpreted as low, moderate, and high between-trial heterogeneity, although interpretation of I 2 depends on the size and number of trials included. We did not plan to use funnel plots to evaluate publication bias and other biases related to a small sample because we expected to include only a few studies. For the same reason, we refrained from any stratified analyses according to differences in patient selection, type of SSRI, treatment regimen, or design in stratified analyses.
All meta-analyses were performed using Review Manager 5.0 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark).
Results
Of the 1,413 articles initially retrieved ( Figure 1 ), we identified 11 studies that evaluated the health effects of SSRI in depressed patients with cardiovascular diseases. Of those, 4 had to be excluded, 1 trial because of absence of a control group, 1 trial because an active control group receiving other antidepressants was used, 1 cohort study because of misclassification of SSRI drugs (venlafaxine and clomipramine were considered SSRIs), and 1 case–control study because it did not include any of the outcomes considered. Seven reports on 6 studies were eligible and included in the analyses. Search in trial registers and screening of references did not result in the identification of additional studies. Four studies concerned RCTs. One trial randomized 50 patients before assessing inclusion criteria and then limited analysis to 17 patients with depression. It was therefore considered incorrectly randomized (thus, observational) and excluded from the main analysis. Another study was a reanalysis of data of a subset of patients participating in an RCT evaluating the effects of cognitive behavior therapy supplemented with SSRI when indicated. The investigators provided estimates adjusted for the main potential confounders, but the intake of SSRI, another antidepressant, or no antidepressant was not properly randomized across subgroups, and groups differed on some prognostic variables. This study was therefore considered observational and excluded from the main analysis. Two of the latter studies, however, were included in the overall analysis, in which all available information were considered. There were no unpublished data.
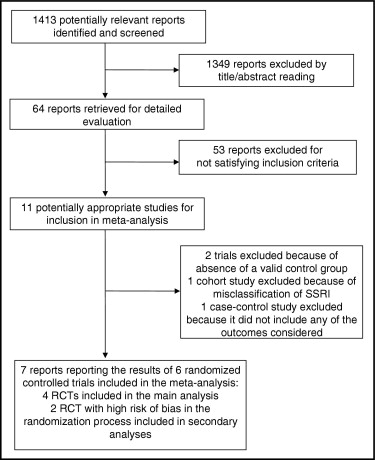
Overall, data were available on 2,461 patients from North America, Europe, Australia, and India, with a mean age that was similar in all trials and close to 58 years ( Table 1 ). Inclusion criteria varied, although all patients were previously admitted for CHD. Depression was established using validated methods in all studies, which had been performed from 1994 through 2006, with a mean follow-up duration varying from a minimum of 12 weeks to a maximum of 29 months. Table 2 presents methodologic characteristics of the included studies. Only Lespérance et al reported the use of adequate methods of sequence generation and concealment of allocation. In a study by Taylor et al, we judged the groups comparable at baseline for confounding factors, except for age and gender. Taylor et al did not model according to the original design, in which patients were randomized to cognitive behavior therapy or usual care, which is a limitation in their analysis. However, they adequately adjusted the analysis for all relevant confounders except gender. Most studies reported adequate blinding of patients. Although trials by Strik et al and Mohapatra et al were described as double-blinded trials, it was unclear whether therapists and/or outcome assessors were blinded. None of the trials described placebo as indistinguishable from SSRI. All but 2 trials performed analysis according to the intention-to-treat principle for the main outcomes of interest. However, Strik et al used end-point analysis for responder criteria, where 18 of 27 controls (67%) and 22 of 27 experimental subjects (81%) were analyzed at 25 weeks. In the observational analysis of Taylor et al, all 1,689 patients meeting inclusion criteria were accounted for in the analyses.
| Study | Relevant Outcomes** | Study Year | Country | Design | Main Inclusion Criteria | Mean Age (years) | Sample Size | Intervention | Dose in Each Arm | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||||
| Strik et al | decrease in depression symptoms (HAMD-17), response after therapy (HAMD-17), remission after therapy (HAMD-17 <7) | 1994–1997 | Netherlands | RCT, double blind | 3–12 months after MI with DSM-III-R criteria for major depressive episode met and HAMD-17 >17 | 56 | 54 | fluoxetine vs placebo | flexible dose 20–40 or 60 mg/day depending on clinical response, mean 47.3 ± 19.1 | 9 or 25 weeks |
| McFarlane et al | decrease in depression symptoms (IDD), all-cause mortality | 1996–1999 | Canada | RCT, double blind | after discharge for MI, scoring ≥15 (2 times) on IDD: minor or major depression | 62 | 27 | sertraline vs placebo | fixed dose 50 mg/day | 6 months |
| Glassman et al | decrease in depression symptoms (HAMD-17 and CGI-I), remission after therapy (CGI-I ≤ 2), readmission for CHD, all-cause mortality | 1997–2001 | 7 countries in Europe, North America, and Australia | RCT, double blind | after discharge for MI (74%) or unstable angina (26%) with DSM-IV criteria for major depression met and BDI >10 | 57 | 369 | sertraline vs placebo | flexible dose 50–200 mg/day depending on clinical response, mean 68.8 ± 40.1 | 24 weeks |
| Lespérance et al | decrease in depression symptoms (HAMD-17), response after therapy (HAMD-24), remission after therapy (HAMD-24 <9), readmission for CHD, all-cause mortality | 2002–2006 | Canada | RCT, double blind | after discharge for CHD with DSM-IV criteria for major depression met for ≥4 weeks and HAMD-24 ≥20 | 58 | 284 | 4 arms: psychotherapy + citalopram, psychotherapy + placebo, citalopram, placebo | flexible dose 20–40 mg/day, mean 33.5 ± 10.4 | 12 weeks |
| Observational or randomized controlled trial with high risk of bias in randomization process | ||||||||||
| Taylor et al | decrease in depression symptoms (HAMD-17, recorded but not reported), readmission for CHD, all-cause mortality | 1996–1999 | United States | RCT, single blind | after admission for acute MI with DSM-IV criteria for depression met for ≥4 or ≥7 d if previous major depression, and HAMD-17 >24 or change in BDI <50% in first 5 weeks | 61 | 1,689 | sertraline (49.5%) or paroxetine (29%), or fluoxetine (13%) or another SSRI (8.6%) vs no intervention | flexible-dose, i.e., sertraline, 50–200 mg/day depending on clinical response | minimum 18 month, mean 29 |
| Mohapatra et al | decrease in depression symptoms (HAMD-17), remission after therapy (HAMD-17 ≤7), re-admission for CHD, all-cause mortality | not reported | India | RCT, single blind | after MI, during hospital stay with DSM-IV criteria for depression | 58 | 38 | sertraline vs no intervention | flexible-dose 50–200 mg/day depending on clinical response | 6 months |
| Adequate Sequence Generation? | Allocation Concealment? | Adequate Blinding of Patients? | Adequate Blinding of Therapists? | Adequate Blinding of Outcome Assessors? | Intention-to-Treat Analysis Performed? | |
|---|---|---|---|---|---|---|
| Strik et al, 2000 | ? | ? | + | ? | ? | + |
| McFarlane et al, 2001 | ? | ? | + | ? | ? | − |
| Glassman et al, 2002 | ? | ? | + | ? | + | + |
| Taylor et al, 2005 ⁎ | − | − | + | |||
| Mohapatra et al, 2005 | ? | ? | ? | ? | + | − |
| Lespérance et al, 2007 | + | + | + | + | + | + |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


