Angioedema is a rare, potentially life-threatening adverse event of renin–angiotensin system inhibitors. The objective of the present study was to determine the risk of angioedema from randomized clinical trials. A PubMed/CENTRAL/EMBASE search was made for randomized clinical trials from 1980 to October 2011 in patients on angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or direct renin inhibitor (DRI). Trials with a total number of patients ≥100 and a duration of ≥8 weeks were included for analysis. Incidence of angioedema was pooled by weighing the incident rate of each trial by the inverse of the variance. Twenty-six trials with 74,857 patients in the ACE inhibitor arm with 232,523 person-years of follow-up, 19 trials with 35,479 patients on ARB with 122,293 person-years of follow-up, and 2 trials with 5,141 patients on DRI with 1,735 person-years of follow-up met the inclusion criteria and were included in the analysis. In head-to-head comparison in 7 trials, risk of angioedema with ACE inhibitors was 2.2 times higher than with ARBs (95% confidence interval [CI] 1.5 to 3.3). With ACE inhibitors and ARBs, incidence of angioedema was higher in heart failure trials compared to hypertension or coronary artery disease trials without heart failure (p <0.0001). Weighted incidence of angioedema with ACE inhibitors was 0.30% (95% CI 0.28 to 0.32) compared to 0.11% (95% CI 0.09 to 0.13) with ARBs, 0.13% (95% CI 0.08 to 0.19) with DRIs, and 0.07% with placebo (95% CI 0.05 to 0.09). In conclusion, incidence of angioedema with ARBs and DRI was <1/2 than that with ACE inhibitors and not significantly different from placebo. Incidence of angioedema was higher in patients with heart failure compared to those without heart failure with ACE inhibitors and ARBs.
In one instance, possibly in two, death resulted from a sudden oedema glottides. —William Osler, MD
Angioedema is a potentially life-threatening but rare adverse event in patients treated with renin–angiotensin system (RAS) inhibitors. RAS inhibitors are extensively prescribed for treatment of hypertension and for cardiovascular and renal protection in patients with heart failure, chronic kidney disease, and at high risk of cardiovascular events. Angioedema seems to be most common with angiotensin-converting enzyme (ACE) inhibitors and occur in 0.1% to 0.5% of patients taking these drugs, although it appears to be more common in African-Americans. Angioedema affects about 1 of 2,500 patients during the first week of exposure. However, it can first appear from a few hours to 8 years after an ACE inhibitor is initiated. The subsequent incidence of angioedema with ACE inhibitors is around 1 in 500 patients per year. Among all instances of angioedema, about 20% are life threatening, affecting the larynx and upper respiratory tract. Among these, about 20% are fatal unless intubated. Because 20 to 30 million patients are on these drugs worldwide, 1 can estimate the total number of fatalities related to angioedema to be around 1,000 per year. Several trials also have reported angioedema using angiotensin receptor blockers (ARBs), although the risk appears to be lower than with ACE inhibitors. The objective of the present study was to determine the risk of angioedema with various RAS inhibitors as reported in prospective clinical trials.
Methods
A systematic literature search was done using subject terms “ACE inhibitors,” “angiotensin receptor blockers,” “direct renin inhibitors,” “aliskiren,” and “angioedema” and using the names of individual ACE inhibitors and ARBs in the PubMed, CENTRAL, and EMBASE databases to identify articles from 1980 through October 2011. The search was limited to studies in human subjects in peer-reviewed journals. There was no language restriction for the search. Reference lists of identified articles, meta-analyses, and bibliographies of original articles were also reviewed. Authors of individual articles were contacted if data were not available. To be included in the analysis, a trial had to fulfill the following criteria: (1) randomized clinical trial comparing ACE inhibitors, ARBs, or direct renin inhibitors (DRIs) to other antihypertensive drugs (including placebo) or to each other; (2) trials that enrolled ≥100 patients; (3) mean duration of study of ≥8 weeks; and (4) information about angioedema. Studies in which ACE inhibitors or ARBs were added as a second- or third-line agent were excluded from the analysis.
Two reviewers (A.K. and R.S.B.) extracted the data independently and in duplicate. Disagreements were resolved by arbitration (J.R. or O.W.P.) and consensus was reached after discussion. We extracted baseline demographics, duration of intervention, cohort of patients enrolled, and outcome of interest for our analysis.
Quality of studies was assessed using methods recommended by the Cochrane Collaboration tools for assessing risk of bias based on 7 components. For each component, studies were described as a low, high, or unclear risk of bias. Studies with 7 of 7 components were considered as low-risk, 6 of 7 components as intermediate risk, and <6 of 7 components as high-risk for bias.
Statistical analysis was done in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using Review Manager (RevMan) [Computer program] (Version 5.0.23 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.) Incidence of angioedema was pooled separately for ACE inhibitors, ARBs, DRIs, and all their comparisons by weighing the incident rate of each trial by the inverse of the variance. Head-to-head comparison was made between ACE inhibitors and ARBs where data were available. Heterogeneity was assessed using I 2 statistics. I 2 is the proportion of total variation observed between trials attributable to differences between trials rather than to sampling error (chance) and we considered an I 2 <25% as low and an I 2 >75% as high. Effect sizes were calculated by the Peto method because it is regarded as the most optimal approach for analysis with fewer events in individual trials. Publication bias was estimated visually by funnel plots and/or using the Begg test and the weighted regression test of Egger et al.
Subgroup analysis was performed for incidence of angioedema for ACE inhibitors and ARBs based on (1) baseline patient population in the study (black vs nonblack), (2) cohort of patients enrolled (heart failure vs nonheart failure), (3) risk of bias in a trial, and (4) duration of study (≤1 vs >1 year). Comparison was also made between patients on ARBs with a history of ACE inhibitor intolerance and those without exposure to an ACE inhibitor. We estimated differences between subgroups according to tests of interaction.
Results
In total, 3,545 articles were identified by the search criteria, of which 221 full-text articles were assessed for eligibility. Forty trials enrolling 206,596 participants (mean age 62 years, 61% men) and a mean follow-up duration of 123 weeks met the inclusion criteria ( Figure 1 ) . Twenty-seven trials were excluded because of lack of data on angioedema with ACE inhibitor or ARB therapy. An additional 7 trials were excluded because an ACE inhibitor or an ARB was added as a second- or third-line agent for therapy.
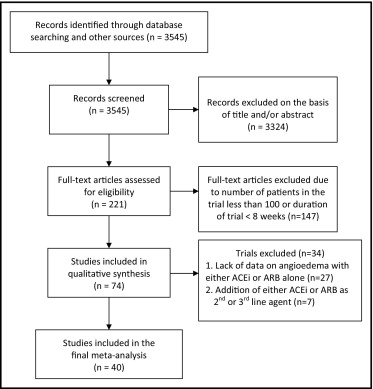
Of the 40 trials that were included, 26 trials compared ACE inhibitors to other antihypertensive drugs (including placebo) with 74,857 patients in the ACE inhibitor arm (mean age 63 years, 64% men) and 232,523 person-years of follow-up; 19 trials with 35,479 patients (mean age 61 years, 59% men) and 122,293 person-years of follow-up were included in the ARB group; and 2 trials with 5,141 patients and 1,735 person-years of follow-up were in the DRI arm. There were 7 trials with 34,381 patients comparing ACE inhibitors head to head with ARBs ( Table 1 ).
| Trial | Patient Characteristics | Total Number of Patients | Follow-Up (weeks) | Mean Age (years) | Men (%) | Black (%) | ACE Inhibitor/ARB |
|---|---|---|---|---|---|---|---|
| Trials with angiotensin-converting enzyme inhibitors | |||||||
| AASK, 2002 | African-American with hypertensive renal disease | 1,094 | 208 | 55 | 61 | 100 | Ramipril vs amlodipine and metoprolol |
| ACCOMPLISH, 2008 | HTN at high risk of CV events | 11,506 | 156 | 68 | 61 | 12.3 | Benazepril + amlodipine vs benazepril + HCTZ |
| ALLHAT, 2002 | ≥55 years old hypertensive with 1 additional risk factor for CHD events | 33,357 | 256 | 67 | 53 | 35.5 | Lisinopril vs amlodipine and chlorthalidone |
| BENEDICT, 2004 | HTN, type 2 DM, normal urinary albumin excretion | 1,204 | 187 | 63 | 54 | NR | Trandolapril vs verapamil vs verapamil + trandolapril vs placebo |
| Cesari et al (TRAIN), 2009 | High-risk CV profile | 290 | 52 | 66 | 57 | 23.8 | Fosinopril vs placebo |
| CHARM-Added, 2003 | CHF class II to IV with EF <40% | 2,548 | 177 | 64 | 79 | 4.9 | Any ACE inhibitor + placebo vs ACE inhibitor + candesartan |
| Cleland et al (PEP-CHF), 2006 | ≥70 years old with diastolic HF | 850 | 109 | 76 | 45 | NR | Perindopril vs placebo |
| DIABHYCAR, 2004 | Type 2 DM with proteinuria or microalbuminuria | 4,912 | 208 | 65 | 70 | NR | Ramipril vs placebo |
| HOPE, 2000 | CVD risk factors | 9,297 | 260 | 66 | 53 | NR | Ramipril vs placebo |
| Keane et al, 1997 | HTN with chronic renal insufficiency | 317 | 106 | 49 | 61 | NR | Enalapril vs control |
| MacLean et al, 1990 | HTN | 128 | 24 | 51 | 58 | 8 | Enalapril vs nifedipine |
| OCTAVE, 2004 | HTN | 25,302 | 24 | 57 | 52 | 10 | Enalapril vs omapatrilat |
| OVERTURE, 2002 | Severe chronic HF and EF <30% | 5,770 | 62 | 63 | 79 | NR | Enalapril vs omapatrilat |
| PEACE, 2004 | Stable CVD with normal or slightly decreased EF | 8,290 | 250 | 65 | 82 | NR | Trandolapril vs placebo |
| Pepine et al (QUASAR), 2003 | CAD with stable angina | 336 | 16 | 65 | 80 | NR | Quinapril high vs low dose |
| PHYLLIS, 2004 | HTN and hypercholesterolemia | 508 | 135 | 58 | 41 | NR | Fosinopril vs HCTZ |
| PREAMI, 2006 | Patients with acute MI | 1,252 | 52 | 73 | 65 | NR | Perindopril vs placebo |
| PROGRESS, 2001 | Previous stroke or TIA | 6,105 | 203 | 64 | 70 | NR | Perindopril vs placebo |
| SOLVD, 1996 | LV systolic dysfunction | 6,769 | 172 | 60 | 84 | NR | Enalapril vs placebo |
| Trials with angiotensin receptor blockers | |||||||
| ALPINE, 2003 | Newly diagnosed HTN | 392 | 52 | 55 | 48 | NR | Candesartan vs HCTZ |
| CHARM-Alternative, 2003 | HF with EF <40% | 2,028 | 147 | 67 | 68 | 2.8 | Candesartan vs placebo |
| DIRECT, 2008 | Type 1 DM | 3,326 | 166 | 31 | 58 | NR | Candesartan vs placebo |
| HEAAL, 2009 | HF | 3,846 | 244 | 66 | 71 | 1 | Losartan 50 vs 150 mg |
| IDNT, 2001 | Diabetic nephropathy and HTN | 1,715 | 135 | 59 | 67 | 13 | Irbesartan vs amlodipine vs placebo |
| LIFE, 2002 | HTN and LVH | 1,195 | 244 | 67 | 57 | 6 | Losartan vs atenolol |
| McGill et al, 2001 | HTN | 818 | 8 | 53 | 60 | 28.2 | Telmisartan vs placebo and HCTZ |
| MITEC, 2009 | HTN + DM | 209 | 156 | 60 | 64 | NR | Candesartan vs amlodipine |
| ROADMAP, 2011 | Type 2 DM and normal albuminuria | 4,447 | 166 | 58 | 46 | NR | Olmesartan vs placebo |
| TRANSCEND, 2008 | Intolerant to ACE inhibitor with CVD or DM with end-organ damage | 5,926 | 56 | 67 | 57 | 1.7 | Telmisartan vs placebo |
| TROPHY, 2006 | Pre-HTN | 787 | 208 | 48 | 60 | 12 | Candesartan vs placebo |
| Volpe et al, 2003 | HTN | 857 | 18 | 68 | 34 | NR | Losartan vs amlodipine |
| Trials comparing angiotensin-converting enzyme inhibitors to angiotensin receptor blockers | |||||||
| ELITE, 1997 | HF with EF <40% | 722 | 47 | 72 | 67 | 4.7 | Captopril vs losartan |
| Himmelmann et al, 2001 | Mild to moderate HTN | 395 | 8 | 59 | 55 | 0.8 | Enalapril vs candesartan |
| Karlberg et al, 1999 | Elderly patients with primary HTN | 278 | 26 | 71 | 42 | NR | Enalapril vs telmisartan |
| Neutel et al, 1999 | Mild to moderate HTN | 578 | 52 | 54 | 66 | 17 | Lisinopril vs telmisartan |
| ONTARGET, 2008 | CVD or DM with end-organ damage | 25,620 | 243 | 66 | 73 | 2.4 | Ramipril vs telmisartan and ramipril + telmisartan |
| OPTIMAAL, 2002 | Acute MI and evidence of HF | 5,477 | 140 | 67 | 71 | 0 | Captopril vs losartan |
| VALIANT, 2003 | Acute MI with HF | 14,703 | 107 | 65 | 69 | 2.8 | Captopril vs valsartan |
| Trials with aliskiren | |||||||
| ACCELERATE, 2011 | Essential HTN | 1,254 | 32 | 58 | 49 | 5 | Aliskiren vs amlodipine vs aliskiren + amlodipine |
| White et al, 2010 | HTN | 12,188 | 16 | 56 | 59 | 8.2 | Aliskiren vs HCTZ, ACE inhibitor, ARB, aliskiren + ARB and placebo |
Of 26 included studies that investigated ACE inhibitors, 18 studies reported adequate generation of an allocation sequence and adequate allocation concealment, and 20 reported adequate masking of participants, personnel, and outcome assessors. From the quality assessment, 18 were deemed as low-bias risk trials and the rest as high-bias risk. Similarly with ARBs, 14 studies were deemed as low-bias risk trials and the rest as high risk.
Patients were screened before randomization (run-in phase) in 15 studies with ACE inhibitors and 12 studies with ARBs. Three of 7,488 patients (0.04%) developed angioedema during the run-in-phase of those treated with enalapril in the Studies of Left Ventricular Dysfunction (SOLVD) trial, 40 of 29,019 patients (0.14%) treated with any of the 3 drug regimens of ramipril, telmisartan, or combination of ramipril and telmisartan developed angioedema in the Ongoing telmisartan Alone and in Combination with Ramipril Global End Point Trial (ONTARGET) before randomization, and 1 of 7,121 patients (0.01%) developed angioedema of those treated with perindopril in the Perindopril in Perindopril Protection against Recurrent Stroke Study (PROGRESS) trial. In studies in patients with previous ACE intolerance, 39 of 1,013 patients (3.8%) in the candesartan group had a history of angioedema with ACE inhibitors in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Alternative trial, 75 of 5,926 patients (1.3%) had a history of angioedema with ACE inhibitors in the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trial.
Of 74,857 patients on ACE inhibitors, 394 developed angioedema during a mean duration of 129 weeks with a weighted incidence of 0.30% (95% confidence interval [CI] 0.28 to 0.32). Of 35,479 patients on ARBs, 52 developed angioedema during a mean duration of 120 weeks with a weighted incidence of 0.11% (95% CI 0.09 to 0.13). Of 5,141 patients on aliskiren, 7 developed angioedema during a mean duration of 24 weeks with an incidence of 0.13% (95% CI 0.07 to 0.19). Incidence of angioedema was highest with omapatrilat (1.7%; which never made it to market), followed by placebo (0.07%), thiazides (0.05%), and calcium channel blockers (0.03%; Figure 2 ) .
Head-to-head comparison between ACE inhibitors and ARBs was available in 7 trials. Risk of angioedema with ACE inhibitor therapy was 2.2 times higher than with ARB therapy (95% CI 1.50 to 3.34, p <0.0001; Figure 3 ) . There was no heterogeneity and the test for bias was negative (p = 0.82, Egger test).
Head-to-head comparison between ACE inhibitors and placebo was available in 10 trials ( Figure 4 ) . Risk of angioedema with ACE inhibitor therapy was 2.8 times higher than with placebo (95% CI 1.63 to 4.79, p = 0.0002). In 7 trials comparing ARBs to placebo, there was no significant difference in risk of angioedema (95% CI 0.39 to 3.61, p = 0.77). There was no heterogeneity between the 2 groups. Thus, fixed effects model was used for analysis (p = 0.76, Egger test).
Incidence of angioedema in black patients (0.92%) on ACE inhibitors was 2 times as high as in nonblack patients (0.44%, p <0.0001). Similarly, incidence of angioedema in patients with heart failure (0.49%) was 1.8 times higher compared to patients without heart failure (0.27%, p <0.0001). In low-risk bias trials, incidence of angioedema (0.27%) was lower than in those with high-risk bias (0.67%, p <0.0001). In studies with duration ≤1 year, incidence of angioedema (0.64%) was around 2.3 times higher compared to studies with >1 year duration (0.27%, p <0.0001; Table 2 ).
| Subgroup | Number of Studies | Number of Patients | Weighted Incidence of Angioedema | Ratio of RR (95% CI) | Interaction p Value |
|---|---|---|---|---|---|
| Angiotensin-converting enzyme inhibitors | |||||
| Black | 3 | 4,893 | 0.92% | 2.05 (1.68–2.49) | <0.0001 |
| Nonblack | 3 | 19,964 | 0.44% | ||
| Heart failure | 7 | 15,974 | 0.49% | 1.81 (1.58–2.08) | <0.0001 |
| Nonheart failure | 19 | 58,883 | 0.27% | ||
| Risk of bias | <0.0001 | ||||
| Low | 18 | 61,047 | 0.27% | 0.40 (0.36–0.44) | |
| High | 8 | 13,810 | 0.67% | ||
| Duration | <0.0001 | ||||
| ≤1 year | 9 | 15,445 | 0.64% | 2.32 (2.11–2.53) | |
| >1 year | 17 | 59,412 | 0.27% | ||
| Angiotensin receptor blockers ⁎ | |||||
| Heart failure | 6 | 12,852 | 0.29% | 3.16 (2.20–4.53) | <0.0001 |
| Nonheart failure | 13 | 22,627 | 0.09% | ||
| Risk of bias | |||||
| Low | 14 | 34,387 | 0.12% | 0.38 (0.14–0.96) | 0.04 |
| High | 5 | 1,092 | 0.32% | ||
| Duration | |||||
| ≤1 year | 7 | 1,909 | 0.31% | 2.81 (1.34–5.92) | 0.006 |
| >1 year | 12 | 33,570 | 0.11% | ||
| Angiotensin-converting enzyme inhibitor intolerance | |||||
| Yes | 3 | 7,801 | 0.11% | 1.05 (0.65–1.68) | 0.84 |
| No | 16 | 27,678 | 0.11% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


