Dabigatran has been associated with greater risk of myocardial infarction (MI) than warfarin. It is unknown whether the increased risk is unique to dabigatran, an adverse effect shared by other oral direct thrombin inhibitors (DTIs), or the result of a protective effect of warfarin against MI. To address these questions, we systematically searched MEDLINE and performed a meta-analysis on randomized trials that compared oral DTIs with warfarin for any indication with end point of MIs after randomization. We furthermore performed a secondary meta-analysis on atrial fibrillation stroke prevention trials with alternative anticoagulants compared with warfarin with end point of MIs after randomization. A total of 11 trials (39,357 patients) that compared warfarin to DTIs (dabigatran, ximelagatran, and AZD0837) were identified. In these trials, patients treated with oral DTIs were more likely to experience an MI than their counterparts treated with warfarin (285 of 23,333 vs 133 of 16,024, odds ratio 1.35, 95% confidence interval 1.10 to 1.66, p = 0.005). For secondary analysis, 8 studies (69,615 patients) were identified that compared warfarin with alternative anticoagulant including factor Xa inhibitors, DTIs, aspirin, and clopidogrel. There was no significant advantage in the rate of MIs with the use of warfarin versus comparators (odds ratio 1.06, 95% confidence interval 0.85 to 1.34, p = 0.59). In conclusion, our data suggest that oral DTIs were associated with increased risk of MI. This increased risk appears to be a class effect of these agents, not a specific phenomenon unique to dabigatran or protective effect of warfarin. These findings support the need for enhanced postmarket surveillance of oral DTIs and other novel agents.
The use of the oral direct thrombin inhibitor (DTI), dabigatran, was associated with lower rates of stroke and systemic embolism compared with warfarin demonstrated in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial. The study also revealed that a higher rate of clinical myocardial infarction (MI) was observed with dabigatran than with warfarin that was found to be statistically not significant in the post hoc analysis. A recent meta-analysis revealed that the rate of coronary events was increased with the use of dabigatran compared with various types of controls. Ximelagatran, another oral thrombin inhibitor no longer available for clinical use, was associated with a significantly increased risk of myocardial ischemia compared with warfarin in patients who had acute deep vein thrombosis. It has been proposed that warfarin might provide a protective effect against MI compared with non-warfarin anticoagulants in patients with atrial fibrillation who are prescribed anticoagulation for stroke thromboprophylaxis. The purpose of this study was twofold. The first objective was to investigate whether the excess rate of MIs with dabigatran compared with warfarin was related to the effect of dabigatran alone or was an effect consistent for the entire class of oral DTIs. The second objective was to evaluate whether the use of warfarin indeed is associated with less incidence of MI in the more recent large-scale atrial fibrillation stroke prevention trials compared with non-warfarin anticoagulants.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. To reduce the signal-to-noise ratio, a deliberate decision was made to include only the data on “myocardial infarction” as compared with “acute coronary syndrome” or other coronary ischemia related terms that are more likely to be influenced by subjective interpretation because detailed description of clinical history at the time of initial presentation or information on electrocardiographic changes, cardiac enzyme levels, and the result of possible coronary ischemia evaluation was not disclosed in the individual published trials. Based on the objectives of the study, we performed 2 separate systematic literature searches.
To address the observation of incidence of MI in trials with oral DTIs, we searched MEDLINE, Embase, and the Cochrane Collaboration, up to March 2013 using key words: “oral,” “direct thrombin inhibitor” with the limiting term “clinical trial” without language or publication date restrictions. We then performed a secondary search using the individual agent name: “dabigatran” with the limiting term “clinical trial,” “ximelagatran” with the limiting term “clinical trial,” and lastly “AZD0837” and “sofigatran” with no limitations. Studies were included in meta-analysis if (1) the comparison between an oral DTI and warfarin was made for any indication and (2) the occurrence of MI after randomization was reported by investigators by any definition.
Regarding the possible protective effect of warfarin in preventing MI in more recent atrial fibrillation stroke prevention trials, we performed a MEDLINE search using the PubMed medical subject heading database using terms: “atrial fibrillation,” “stroke prevention and control,” “anticoagulants/therapeutic use,” and “warfarin/therapeutic use” with the limiting term of “randomized trials” and time limit of “10 years.” Studies were included in the meta-analysis if (1) comparison between warfarin and any alternative antithrombotic agent was performed and (2) the occurrence of MI after randomization was reported by investigators by any definition.
The data were analyzed using Comprehensive Meta-Analysis, version 2 (Biostat, Englewood, New Jersey). The pooled odds ratio (OR) with 95% confidence interval (CI) for MI was calculated using the fixed-effects Mantel-Haenszel model, whereas in the case of significant heterogeneity across studies, the random-effects model was used instead. The likelihood of statistical heterogeneity was assessed using the Cochrane Q test and quantified with the I² statistic. An I² >50% was considered statistically significant heterogeneity. In addition, an influence analysis, in which meta-analysis estimates are computed omitting 1 study at a time, was performed for the primary end point. Publication bias was evaluated with the funnel plot and the Egger regression test.
Results
The results of the literature search on oral DTIs versus warfarin are shown in Figure 1 . A total of 676 publications were identified and screened. Of those, 14 full-text manuscripts were found eligible for detail review. Eleven randomized controlled trials fulfilled the inclusion criteria and were selected for meta-analysis, with the total number of 39,357 participants. The individual study details are listed in Table 1 . The MI definition was prespecified in the trial design in less than half of the studies. None of the studies had prespecified definition of other ischemic coronary terms such as “acute coronary syndrome,” “ischemic coronary event,” or “unstable angina.” Overall, the entire cohort of oral DTIs was associated with a significantly higher rate of MI compared with warfarin. MI was reported in 285 of 23,333 subjects treated with oral DTIs versus 133 of 16,024 subjects treated with warfarin, with an OR of 1.35 (95% CI 1.10 to 1.66), p = 0.005 using the fixed-effects model. There was no significant heterogeneity among the studies (Q = 15.3, degree of freedom [DF] = 9, I 2 = 41.1% and P = 0.084 for heterogeneity; Figure 2 ). Among the 4 trials that compared dabigatran to warfarin, there were significantly higher rates of MI among those randomized to dabigatran. These included 212 out of 14,954 subjects treated with dabigatran versus 78 out of 8,803 subjects treated with warfarin (OR 1.41, 95% CI 1.09 to 1.83, p = 0.009, Q = 4.5, DF = 3, I 2 = 33.2% and P = 0.21 for heterogeneity).
| Study Name | Population | Design | Primary End Point | Number of Subjects | Prespecified MI Definition ∗ | Thrombin Inhibitor † | Duration (Mo) |
|---|---|---|---|---|---|---|---|
| RE-LY | Atrial fibrillation | Noninferiority open label | Stroke and SE | 18,113 | Yes | Dabigatran 150 and 110 mg BID | 24 |
| RE-COVER | Acute VTE | Noninferiority double blind | VTE or related death | 2,539 | No | Dabigatran 150 mg BID | 6 |
| RE-MEDY | VTE | Noninferiority double blind | VTE or related death | 2,866 | ‡ | Dabigatran 150 mg BID or placebo | 18 |
| RE-ALIGN § | Mechanical valves | Phase II, open label | Mortality and morbidity | 249 ‖ | No ¶ | Dabigatran 150, 220, and 300 mg BID | 3 |
| SPORTIF III | Atrial fibrillation | Noninferiority open label | Stroke and SE | 3,410 | Yes | Ximelagatran 36 mg BID | 17.4 |
| SPORTIF V | Atrial fibrillation | Noninferiority double blind | Stroke and SE | 3,922 | Yes | Ximelagatran 36 mg BID | 20 |
| THRIVE | Acute VTE | Noninferiority double blind | Recurrent VTE | 2,489 | No | Ximelagatran 36 mg BID | 6 |
| EXULT A and B | Thromboprophylaxis | Superiority double blind | VTE and all-cause death | 3,800 | No | Ximelagatran 24 and 36 mg BID | 1–1.5 |
| Lip et al | Atrial fibrillation | Tolerability and dose guiding | Bleeding and adverse events | 955 | No | AZD0837, 150 to 450 mg QD and 200 mg BID | 5 |
| Olsson et al | Atrial fibrillation | Safety and tolerability | Bleeding and adverse events | 249 | No | AZD0837, 150 and 300 mg BID | 3 |
∗ The original study publication and additional publications including supplementary appendices and trial design publications were screened for prespecified definition of MI or any related terms as any type of end point or an adverse event after randomization.
† Comparison in this table was against adjusted dose warfarin to an international normalized ratio of 2.0-3.0.
‡ As per study protocol, all suspected acute coronary syndromes (ACS) were evaluated centrally and blindly by ACS Adjudication Committee. The operating procedures containing all details regarding ACS will be available as a separate document. This document is currently not available in the public domain.
§ http://www.fda.gov/Drugs/DrugSafety/ucm332912.htm . The study had blinded end point that also included dabigatran plasma drug levels.
‖ Study was prematurely terminated. The planned study population was 405.
¶ An independent adjudication committee was established for the blinded adjudication of MI among other efficacy outcome end points.
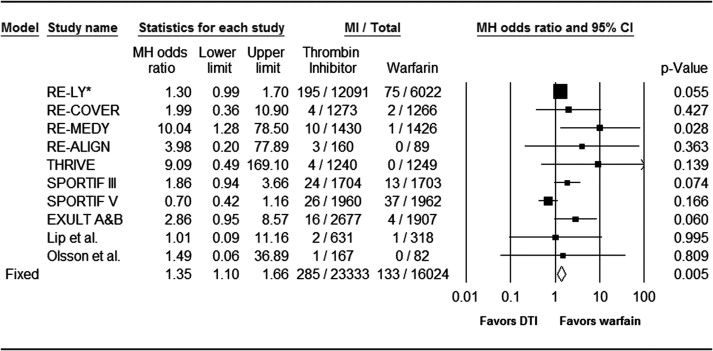
In the 5 trials comparing ximelagatran versus warfarin, there was a trend toward increased rates of MI among those treated with ximelagatran but subgroup meta-analysis did not reach statistical significance. There were 70 out of 7,581 in the ximelagatran group compared with 54 out of 6,821 in the warfarin group (OR 1.23, 95% CI 0.86 to 1.76, p = 0.25, Q = 10.3, DF = 3, I 2 = 70.8% and P = 0.016 for heterogeneity). Among the trials with ximelagatran, the meta-analysis by treatment indication revealed more than 3 times increased risk of MI for those treated with ximelagatran compared with warfarin in the subgroup of venous thromboembolism and thromboprophylaxis. There were 20 MIs out of 3,917 subjects treated with ximelagatran versus 4 out of 3,156 subjects treated with warfarin (OR 3.46, 95% CI 1.25 to 9.57, p = 0.017, Q = 0.53, DF = 1, I 2 = 0.0% and P = 0.46 for heterogeneity).
Among the trials with AZD0837, there was no significant difference between rates of MI among the groups. There were 3 MIs out of 798 subjects treated with AZD0837 versus 1 out of 400 subjects treated with warfarin (OR 1.17, 95% CI 0.17 to 7.94, p = 0.87, Q = 0.036, DF = 1, I 2 = 0.0% and P = 0.85 for heterogeneity).
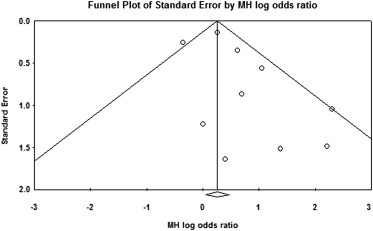
Influence analysis of the entire cohort demonstrated that none of the individual studies appeared to have significant impacts on the overall combined effect sizes ranging from 1.29 to 1.54 (p value ranging from 0.0 to 0.034) for fixed effect. There was no evidence of significant publication bias demonstrated by funnel panel and Egger regression test intercept at 0.90, p = 0.13 (2 tailed) ( Figure 3 ).
The results of the literature search on warfarin versus comparator anticoagulant are shown in Figure 4 . We identified 564 published reports and reviewed 18 full-text manuscripts. Eight randomized controlled trials fulfilled the inclusion criteria and were selected for meta-analysis with the total number of 69,615 participants. The comparator in different studies included aspirin with and without clopidogrel, oral and parenteral factor Xa inhibitors, and oral DTIs. The individual study details are outlined in Table 2 . There was significant heterogeneity among the studies with Q = 15.9, DF = 7, I 2 = 56.1%, P = 0.026. Overall, subjects treated with warfarin had similar rate of MI than those treated with comparator agents. There were 403 out of 31,867 subjects treated with warfarin compared with 503 out of 37,748 subjects treated with comparator agents (OR 1.06, 95% CI 0.85 to 1.34, p = 0.59), using random-effects model ( Figure 5 ). Influence analysis demonstrated that none of the individual studies appeared to have significant impacts on the overall combined effect sizes ranging from 1.01 to 1.14 (p value ranging from 0.31 to 0.92) for random effect. There was no evidence of significant publication bias demonstrated by funnel panel and Egger regression test, intercept at 1.29, p = 0.38 (2 tailed) ( Figure 6 ).
| Study Name | Design | Primary End Point | Number of Participants | Prespecified MI Definition | Comparator Regimen ∗ | Duration (Mo) |
|---|---|---|---|---|---|---|
| AMADEUS | Noninferiority open label | Stroke and SE | 4,576 | No | Idraparinux 2.5 mg SC once weekly | 10.7 |
| ACTIVE W | Noninferiority open label | Stroke and SE, MI and vascular death | 6,706 | Yes | Clopidogrel 75 and aspirin 75–100 mg daily | 15.4 |
| BAFTA | Randomized open label | Fatal or disabling stroke, SE | 937 | No | Aspirin 75 mg daily | 32.4 |
| ROCKET AF | Noninferiority double blind | Stroke and SE | 14,264 | Yes | Rivaroxaban 20 mg daily | 23.2 |
| ARTISTOTLE | Noninferiority double blind | Stroke and SE | 18,201 | No | Apixaban 5 mg twice daily | 21.6 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


