Glycoprotein IIb/IIIa receptor inhibitors (GPIs) have been widely adopted as an adjuvant regimen during primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction, but whether intracoronary administration of these potent antiplatelet agents conveys better efficacy and safety over the intravenous route has not been well addressed. A meta-analysis was performed by a systematic search of the published research for randomized controlled trials comparing intracoronary versus intravenous administration of GPIs in patients with ST-segment elevation myocardial infarction. Eight studies involving 686 patients in the intracoronary arm and 660 in the intravenous arm met the inclusion criteria. Postprocedural Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow (odds ratio [OR] 1.46, 95% confidence interval [CI] 1.08 to 1.98, p <0.05) and myocardial reperfusion grade 2 or 3 (OR 1.78, 95% CI 1.29 to 2.46, p <0.001) were markedly more often achieved in patients who received intracoronary boluses of GPIs than those receiving the intravenous strategy. Intracoronary administration resulted in a reduced incidence of mortality (OR 0.44, 95% CI 0.21 to 0.92, p <0.05), target vessel revascularization (OR 0.53, 95% CI 0.29 to 0.99, p <0.05), and the composite end point of major adverse cardiac events (OR 0.48, 95% CI 0.31 to 0.76, p <0.005) at 30-day follow-up. No significant difference was found in terms of major or minor bleeding (OR 1.14, p = 0.71, and OR 0.86, p = 0.47 respectively). In conclusion, intracoronary administration of GPIs yielded favorable outcomes in postprocedural blood flow restoration and 30-day clinical prognosis in patients with ST-segment elevation myocardial infarction. The intracoronary use of GPIs can be recommended as a preferred regimen during primary percutaneous coronary intervention.
Glycoprotein IIb/IIIa receptor inhibitors (GPIs) are the most potent antiplatelet agents adopted as an adjuvant regimen during primary percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI). On the basis of early success with intracoronary urokinase or heparin use , the potential of localized administration of GPIs has been investigated with great interest. A systematic review by Hansen et al in 2010 argued that intracoronary abciximab reduced major adverse cardiac events (MACEs) in patients with acute coronary syndromes, but the results were not of adequate power, because of the varied clinical settings of the retrospective trials included as well as a limited number of randomized trials available in the analysis. With a thorough elaboration of angiographic and clinical outcomes, we aimed in this meta-analysis of randomized controlled trials to evaluate the efficacy and safety of intracoronary administration of GPIs in patients with STEMI, thus further optimizing the contemporary administration strategy of local antithrombotic approaches.
Methods
We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials for randomized clinical trials published from January 2000 to June 2011. The following key words were used: “intravenous,” “intracoronary,” “glycoprotein IIb/IIIa receptor inhibitor,” “abciximab,” “tirofiban,” “eptifibatide,” “primary PCI,” “myocardial infarction,” “randomized,” and “clinical trials.”
The inclusion criteria were as follows: (1) a diagnosis of STEMI, (2) a randomized trial that assigned patients to either intracoronary or intravenous GPIs before primary PCI, and (3) results reported and made available by the investigators. Regardless of the language or form of the publication, any studies that met these requirements were considered eligible for the meta-analysis. Two independent investigators searched and reviewed the reports and finally identified 8 randomized controlled trials comparing intracoronary with intravenous administration of GPIs in patients with STEMI who underwent primary PCI. The overall search procedure is shown in Figure 1 .
The primary end points were postprocedural Thrombolysis In Myocardial Infarction (TIMI) flow grade and myocardial reperfusion assessed by myocardial blush grade or TIMI myocardial perfusion grade. The secondary end points were mortality and MACEs at 30-day follow-up. MACEs were defined as the composite of death, reinfarction, and target vessel revascularization. A safety end point was major bleeding complications according to TIMI or Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) bleeding classification. In case of unclear or missing data for the end points, ≥2 separate attempts were made to clarify the data by contacting the primary authors.
Two independent investigators (Y.W. and B.W.) performed the study search, selection, abstraction, and appraisal. All outcomes of interest were abstracted on the basis of the protocol definitions of the trials included. We also collected baseline information, such as trial name, first author, year of publication, number of patients enrolled, and major clinical characteristics. Disagreements were resolved by consensus. In case of incomplete or unclear data, attempts were made to obtain clarification by contacting the primary authors. Quality of Reporting Meta-Analyses (QUOROM) guidelines were referred to in our meta-analysis.
Data were processed according to the intention-to-treat principle. Dichotomous variables are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). We assessed for heterogeneity among studies using Cochran’s test and computing the I 2 statistic. We examined publication bias by constructing Begg’s funnel plots and Egger’s plots. The pooled OR was calculated with the Mantel-Haenszel method for fixed effects and the DerSimonian and Laird method for random effects. Finally, sensitivity analyses were conducted by excluding 1 study at a time to assess the contribution of each individual study to the overall treatment effects. Statistical analyses were performed using the RevMan version 5 (The Cochrane Collaboration, Oxford, United Kingdom) and Stata version 9.0 (StataCorp LP, College Station, Texas). Statistical significance was defined as a 2-sided p value <0.05.
Results
A total of 8 randomized controlled trials comparing intracoronary with intravenous administration of GPIs in patients with STEMI were enrolled in our meta-analysis. The baseline characteristics of the included trials are listed in Table 1 . Of 1,346 patients involved, 686 were randomized to the intracoronary group and 660 to the intravenous group. Detailed information about dosage regimens of GPIs is listed in Table 2 . Intracoronary boluses of GPIs were delivered just after restoration of anterograde flow to allow high GPI concentration in the target region. Intravenous GPIs were administered before or during PCI. Patient selection criteria and trial designs were similar among all studies. However, blinded visual assessment of angiographic parameters was performed in 7 of 8 trials. All patients were pretreated with dual-antiplatelet therapy (loading doses of aspirin 300 to 600 mg and clopidogrel 300 to 600 mg) followed by maintenance doses of aspirin 75 to 100 mg/day and clopidogrel 75 mg/day for ≥6 months, except for those in the trial by Bellandi et al, who were treated with intravenous aspirin 500 mg followed by ticlopidine 250 mg twice daily for 4 weeks and aspirin 100 mg/day indefinitely.
| Study | Number of Patients | Mean Age (years) | Men | DM | SH | Previous MI | LAD Culprit Artery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC | IV | IC | IV | IC | IV | IC | IV | IC | IV | IC | IV | IC | IV | |
| Bellandi et al | 22 | 23 | 62 | 61 | 17 (77%) | 18 (78%) | 4 (18%) | 7 (30%) | 10 (46%) | 8 (35%) | 0 | 0 | 10 (46%) | 10 (44%) |
| Yang et al | 28 | 26 | 60 | 57 | 26 (93%) | 17 (65%) | 5 (18%) | 6 (23%) | 13 (46%) | 13 (50%) | 2 (7%) | 1 (4%) | NA | NA |
| LIPSIAbciximab-STEMI | 77 | 77 | 64 | 66 | 63 (82%) | 59 (77%) | 24 (31%) | 22 (29%) | 54 (70%) | 57 (74%) | 8 (10%) | 7 (9%) | NA | NA |
| Dominguez-Rodriguez et al | 25 | 25 | 66 | 70 | 18 (72%) | 20 (80%) | 13 (52%) | 15 (60%) | 14 (56%) | 13 (52%) | NA | NA | 14 (56%) | 16 (64%) |
| CICERO | 271 | 263 | 64 | 64 | 208 (77%) | 187 (71%) | 36 (13%) | 29 (11%) | 119 (44%) | 129 (49%) | 32 (12%) | 23 (9%) | 121 (45%) | 124 (47%) |
| EASY-MI | 53 | 52 | 59 | 59 | 41 (77%) | 43 (83%) | 4 (8%) | 5 (10%) | 23 (43%) | 23 (44%) | 6 (11%) | 9 (17%) | 22 (42%) | 21 (40%) |
| Iversen et al | 185 | 170 | 62 | 62 | 151 (82%) | 135 (80%) | 26 (14%) | 18 (11%) | 73 (40%) | 68 (40%) | NA | NA | 88 (48%) | 86 (51%) |
| Kırma et al | 25 | 24 | 57 | 56 | 23 (92%) | 21 (88%) | 3 (12%) | 5 (21%) | 5 (20%) | 8 (33%) | NA | NA | 15 (60%) | 15 (63%) |
| Study | Trial Design (GPI Administration) | Inclusion Criteria | Primary End Points |
|---|---|---|---|
| Bellandi et al | IC abciximab bolus (0.25 mg/kg) with a subsequent 12-hour abciximab infusion (0.125 μg/kg/min) vs IV | STEMI <6 hours | LVEF, TIMI flow, MBG |
| Yang et al | IC tirofiban bolus (10 μg/kg) with a subsequent 36-hour tirofiban infusion (0.15 μg/kg/min) vs IV | STEMI <12 hours | TIMI flow, TMPG, MACEs |
| LIPSIAbciximab-STEMI | IC abciximab bolus (0.25 mg/kg) with a subsequent 12-hour abciximab infusion (0.125 μg/kg/min) vs IV | STEMI <12 hours | Microvascular obstruction extent, infarct size, |
| Dominguez-Rodriguez et al | IC abciximab bolus (0.25 mg/kg) with a subsequent 12-hour abciximab infusion (0.125 μg/kg/min) vs IV | STEMI <6 hours | TIMI flow |
| CICERO | IC abciximab bolus only (0.25 mg/kg) vs IV abciximab bolus (0.25 mg/kg) plus infusion (12 hours at 0.125 μg/kg/min) | STEMI or new LBBB <12 hours | Complete ST-segment resolution |
| EASY-MI | IC abciximab bolus (0.25 mg/kg) plus infusion (12 hours at 0.125 μg/kg/min) or fixed-dose bolus only (the sum of bolus and infusion amount) vs IV | STEMI or new LBBB <6 hours | Platelet aggregation inhibition 10 minutes after bolus |
| Iversen et al | IC abciximab bolus (0.25 mg/kg) with a subsequent 12-hour abciximab infusion (0.125 μg/kg/min) vs IV | STEMI <12 hours | MACEs |
| Kırma et al | IC tirofiban bolus only (25 μg/kg) vs IV tirofiban bolus (25 μg/kg) plus infusion (18 hours at 0.15 μg/kg/min) | STEMI or new LBBB <12 hours | Microvascular resistance, coronary flow reserve |
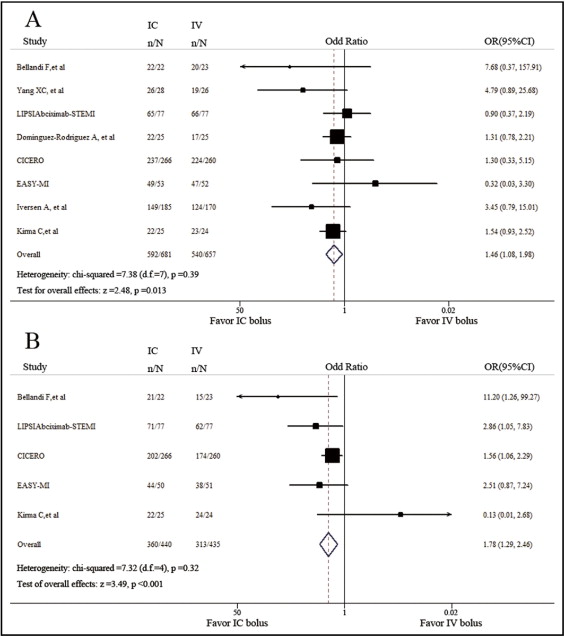
Final TIMI grade 3 flow was achieved in 86.9% and 82.2% of the patients in the intracoronary group and intravenous groups, respectively. By the fixed-effects model, we found that patients benefited more from intracoronary bolus in terms of procedural success rate (TIMI grade 3 flow; OR 1.46, 95% CI 1.08 to 1.98, p <0.05; Figure 2 ). No significant heterogeneity was found among trials (chi-square = 7.38, p = 0.39, I 2 = 5.1%). Myocardial reperfusion in the PCI-targeting area was assessed by myocardial blush grade or TIMI myocardial perfusion grade as the 2 methods were currently preferred to confirm tissue-level myocardial perfusion after primary PCI. Incidence of myocardial blush grade 2 or 3 or TIMI myocardial perfusion grade 2 or 3 was higher in the intracoronary group than in the intravenous group (OR 1.78, 95% CI 1.29 to 2.46, p <0.001; chi-squares = 7.32, p = 0.12, I 2 = 45.3%; Figure 2 ). Excluding 1 study at a time did not have any relevant influence on the overall results of the analyses.
Compared with intravenous bolus of GPI, intracoronary bolus proved to be effective in reducing 30-day mortality (OR 0.44, 95% CI 0.21 to 0.92, p <0.05; chi-square = 2.53, p = 0.64, I 2 = 0%; Figure 3 ) and target vessel revascularization (OR 0.53, 95% CI 0.29 to 0.99, p <0.05; chi-square = 2.05, p = 0.36, I 2 = 2.4%) in patients with STEMI by the fixed-effect model. In addition, a trend toward a decrease in reinfarction at 30-day follow-up in the intracoronary group was observed, but not of statistical significance (OR 0.52, 95% CI 0.23 to 1.19, p = 0.12; chi-square = 0.71, p = 0.87, I 2 = 0%). The composite end point of mortality, reinfarction, and target vessel revascularization was significantly reduced in the intracoronary group (OR 0.48, 95% CI 0.31 to 0.76, p <0.005; chi-square = 5.11, p = 0.16, I 2 = 41.3%; Figure 3 ). When 1 study by Iversen et al focusing on comparing MACEs between intracoronary and intravenous administration was removed, there was still a trend favoring intracoronary over intravenous bolus in reducing 30-day mortality (OR 0.61, 95% CI 0.26 to 1.45, p = 0.26; chi-square = 0.84, p = 0.84, I 2 = 0%) and MACEs (OR 0.66, 95% CI 0.36 to 1.22, p = 0.19; chi-square = 2.74, p = 0.26, I 2 = 26.9%).





