This study sought to investigate the relative safety and efficacy of bivalirudin versus heparin plus glycoprotein (GP) IIb/IIIa inhibitors in patients undergoing percutaneous coronary intervention (PCI) and in those with ST-segment elevation myocardial infarction (STEMI). The safety of bivalirudin in PCI, particularly in patients with STEMI, continues to be debated. We searched the on-line databases for randomized controlled trials of bivalirudin versus heparin plus GP IIb/IIIa inhibitors. Data on study design, inclusion and exclusion criteria, sample characteristics, and clinical outcomes at 30 days were extracted. A total of 19,856 PCI patients included in 7 randomized trials and 5,820 patients with STEMI included in 2 randomized trials were separately analyzed. At 30 days, bivalirudin use in patients undergoing PCI resulted in similar rates of death, myocardial infarction, repeat revascularization, and stent thrombosis. In patients with STEMI, bivalirudin use resulted in decreased cardiac mortality (risk ratio [RR] 0.70, 95% confidence interval [CI] 0.50 to 0.97, p = 0.03) compared with heparin plus GP IIb/IIIa inhibitors but an increase in definite stent thrombosis at 30 days (RR 1.88, 95% CI 1.09 to 3.24, p = 0.02) driven by an increase in acute stent thrombosis (RR 5.48, 95% CI 2.30 to 13.07, p = 0.0001). Bivalirudin use was associated with a decrease in Thrombolysis In Myocardial Infarction (TIMI) major (RR 0.58, 95% CI 0.46 to 0.74, p <0.0001) and TIMI minor (RR 0.55, 95% CI 0.48 to 0.63, p <0.0001) bleeding rates in PCI patients as well as in a subgroup of patients with STEMI. In conclusion, in PCI patients anticoagulation with bivalirudin results in similar ischemic adverse events and a reduction in TIMI major and minor bleeding at 30 days compared with heparin plus GP IIb/IIIa inhibitors. In patients with STEMI, bivalirudin use is associated with a reduction in TIMI major and minor bleeding and fewer deaths from cardiac causes but an increase in acute and 30-day definite stent thrombosis.
Bivalirudin (Angiomax; The Medicines Company, Fort Lee, New Jersey), a direct thrombin inhibitor, is a synthetic polypeptide derived from the native anticoagulant hirudin, with attractive features for utilization during percutaneous coronary intervention (PCI) including short half-life and less monitoring. Multiple randomized trials have compared bivalirudin to heparin or enoxaparin plus glycoprotein (GP) IIb/IIIa inhibitors in patients undergoing PCI or in patients with acute coronary syndrome. Recently, European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial has examined the use of bivalirudin in patients with ST-segment elevation myocardial infarction (STEMI) during emergency transport for primary PCI compared with unfractionated heparin (UFH) and optional GP IIb/IIIa inhibition. This is the second major randomized trial after the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial that compared bivalirudin versus heparin plus routine GP IIb/IIIa inhibition in patients with STEMI. Several meta-analyses and pooled patient-level analyses of randomized trials have previously compared the 2 anticoagulation strategies, with 2 major trials of high-risk patients with non-STEMI and STEMI now being published since the last analysis. We have conducted a comprehensive meta-analysis of all published randomized trials to evaluate the safety and efficacy of bivalirudin compared with heparin or enoxaparin plus GP IIb/IIIa inhibitors in PCI patients and those presenting with STEMI.
Methods
We systematically searched the literature for randomized clinical trials comparing bivalirudin with combined use of heparin or enoxaparin plus GP IIb/IIIa inhibitors. The investigators searched the PubMed, Cochrane Central Register of Controlled Trials, EMBASE, and Cumulative Index to Nursing & Allied Health Literature databases for English language, peer-reviewed publications comparing bivalirudin and heparin or enoxaparin plus GP IIb/IIIa inhibitor use in patients undergoing PCI up to November 2013. We used the following keywords: “bivalirudin,” “heparin,” “glycoprotein IIb/IIIa inhibitor,” “PCI,” “percutaneous coronary intervention,” “acute coronary syndrome,” and “STEMI.” In addition, we manually searched clinical trial databases, reviews, meta-analyses, and the reference lists of all retrieved reports for potential relevant studies not found in our initial electronic database search.
We used the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines for reporting systematic reviews and meta-analyses of randomized controlled trials. The studies were included if they (1) were prospective randomized controlled trials; (2) were published as manuscripts in peer-reviewed journals with full available text in English; (3) included patients undergoing PCI, either elective or urgent; (4) compared subjects receiving either bivalirudin with provisional use of GP IIb/IIIa inhibitors or UFH or enoxaparin plus provisional or routine GP IIb/IIIa inhibitors; and (5) length of follow-up of at least 48 hours and up to 30 days after PCI.
Two investigators (PS and RN) reviewed all the trials, ensured that they met the inclusion criteria, and abstracted the data; disagreements were resolved by consensus. We performed objective assessment of the trials using the methods specified in the Cochrane Handbook for Systematic Reviews of Interventions . Efficacy outcomes reported were all-cause mortality, cardiac death, myocardial infarction (MI), stent thrombosis (ST; acute and 30-day ST), and repeat revascularization. The safety outcomes were reported as per Thrombolysis In Myocardial Infarction (TIMI) criteria, TIMI major bleeding and TIMI minor bleeding ; site bleeding and blood transfusion were extracted and reported as well. ST was reported according to Academic Research Consortium criteria. All end points were reported through 30 days.
We reported risk ratios (RR) and their respective 95% confidence intervals (CI) for each study and for the pooled analysis of all studies comparing bivalirudin and heparin or enoxaparin plus GP IIb/IIIa inhibitors as well as for the studies including only patients with STEMI. We assessed the heterogeneity using the Cochran Q test and the Higgins I 2 test. A Cochran Q p value of <0.10 and I 2 >50% were considered significant to demonstrate heterogeneity in this meta-analysis. Random-effects model described by DerSimonian and Laird was used for the main analysis. Statistical analysis was performed with Review Manager (RevMan, version 5.2.7; The Nordic Cochrane Center, The Cochrane Collaboration, 2012, Copenhagen, Denmark). All the p values were 2-tailed with statistical significance level at 0.05, and CI was calculated to 95%.
Results
We identified 749 reports, of which 28 full-text reports were assessed and reviewed for possible inclusion in the analysis ( Figure 1 ). Our review yielded 7 randomized controlled trials comparing bivalirudin with heparin and GP IIb/IIIa inhibitors in PCI patients, including a total of 19,856 patients. Only 2 trials (HORIZONS-AMI and EUROMAX) examined patients with STEMI, a total of 5,820 patients. Characteristics of each of the individual studies are summarized in Table 1 . Baseline characteristics of patients included, procedures performed, and medications administered in these trials are summarized in Table 2 . Events reported from the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial were abstracted from a subgroup analysis of PCI-only patients. Bivalirudin was primarily used as monotherapy unless GP IIb/IIIa inhibitors had to be used as bailout therapy, which ranged from 0% to 11.5% of patients in included trials. In the EUROMAX trial, the use of GP IIb/IIIa inhibitors with heparin was optional.
| Study | Type of Patients | Total Patients | Study Medications | P2Y 12 Loading Dose (% of Patients) | Heparin Use Prior to Procedure | Outcomes Reported (Days) |
|---|---|---|---|---|---|---|
| CACHET, 2002 | Elective PCI | 268 | Biv + provisional abciximab vs. UFH (bolus 70 IU/kg) + abciximab. | Clopidogrel | NA | 7 |
| REPLACE-2, 2003 | Elective stenting and unstable angina | 6010 | Biv vs. UFH (bolus 65 IU/Kg) + GPI. | Clopidogrel 300 mg (83%–85%) | NA | 30 |
| PROTECT-TIMI 30, 2006 | Unstable angina and NSTEMI | 857 | Biv vs. eptifibatide + UFH (bolus 50 U/kg) or enoxaparin (0.5 mg/kg IV). | Clopidogrel 300 mg in all patients prior to stenting. | NA | 2 |
| ACUITY, 2006 | Unstable angina and NSTEMI | 13819 | Biv alone vs. Biv + GPI vs. UFH (bolus 65 IU/Kg) + GPI. | Clopidogrel (62%–64%) | Biv arm (64%), Hp + GPI arm (65%) | 30 |
| HORIZONS-AMI, 2008 | STEMI | 3602 | Biv vs. UFH (bolus 60 IU/Kg) + GPI. | Clopidogrel 300 mg (33%–35%), 600 mg (61%–63%) | Biv arm (66%), Hp + GPI arm (76%) | 30 |
| ISAR-REACT 4, 2011 | NSTEMI | 1721 | Biv vs. UFH (bolus 70 units/kg) + abciximab. | 600 mg clopidogrel to all patients | NA | 30 |
| EUROMAX, 2013 | STEMI | 2218 | Biv vs. UFH (100 IU/kg without a GPI or 60 IU/kg with a GPI) or IV enoxaparin (0.5 mg/kg). | Clopidogrel (50%–52%), prasugrel (29%–31%), ticagrelor (19%–19.4%) | Biv arm (2.2%), Hp + GPI arm (90%) | 30 |
| Study | CACHET | PROTECT TIMI 30 | ACUITY | REPLACE-2 | HORIZONS-AMI | ISAR-REACT 4 | EUROMAX | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biv | Hp + GPI | Biv | Hp + GPI | Biv | Hp + GPI | Biv | Hp + GPI | Biv | Hp + GPI | Biv | Hp + GPI | Biv | Hp + GPI | |
| N | 144 | 94 | 284 | 573 | 2619 | 2561 | 2994 | 3008 | 1800 | 1802 | 860 | 861 | 1089 | 1109 |
| Age (years) | 64–65.1 | 62.4 | 59.7 | 60 | 63 | 63 | 62.6 | 62.6 | 59.8 | 60.7 | 67.5 | 67.5 | 61 | 62 |
| Male | 105 (73%) | 73 (77%) | (68%) | (66%) | 1919 (73%) | 1860 (73%) | 2236 (75%) | 2229 (74%) | 1388 (77%) | 1372 (76%) | 661 (77%) | 661 (77%) | 814 (75%) | 861 (78%) |
| Diabetes mellitus | NA | NA | (44%) | (36%) | 721/2603 (28%) | 703/2543 (28%) | 840 (28%) | 784 (26%) | 281 (16%) | 312 (17%) | 243 (28%) | 257 (30%) | 127 (12%) | 169 (15%) |
| Hypertension | NA | NA | (65%) | (66%) | 1714/2611 (66%) | 1673/2546 (66%) | 1965 (66%) | 2040 (68%) | 931 (52%) | 993 (55%) | 727 (84%) | 745 (86%) | 459 (42%) | 504 (45%) |
| Hyperlipidemia | NA | NA | (56%) | (54%) | 1436/2566 (56%) | 1409/2519 (56%) | NA | NA | 781 (43%) | 769 (43%) | 580 (67%) | 600 (70%) | 398 (37%) | 417 (38%) |
| Smoking | NA | NA | (36%) | (37%) | 795/2571 (31%) | 770/2507 (31%) | 796 (27%) | 762 (26%) | 845 (47%) | 807 (45%) | 195 (22%) | 215 (25%) | 453 (42%) | 472 (43%) |
| Prior MI | NA | NA | (20%) | (22%) | 798/2563 (31%) | 761/2506 (30%) | 1099 (37%) | 1085 (37%) | 187 (10%) | 205 (11%) | 163 (19%) | 188 (22%) | 80 (7%) | 113 (10%) |
| Prior PCI | NA | NA | (24%) | (25%) | 1030/2596 (40%) | 979/2545 (38%) | 1029 (34%) | 1059 (35%) | 188 (10%) | 198 (11%) | 267 (31%) | 292 (34%) | 97 (9%) | 108 (10%) |
| Prior CABG | NA | NA | (6.5%) | (7%) | 468/2613 (18%) | 442/2555 (17%) | 538 (18%) | 564 (19%) | 59 (3%) | 46 (2.5%) | 89 (10%) | 92 (11%) | 18 (1.7%) | 29 (3%) |
| Creatinine clearance <60 ml/min | NA | NA | NA | NA | 441/2475 (18%) | 457/2407 (19%) | NA | NA | 262 (16%) | 292 (17%) | NA | NA | 147 (15%) | 165 (16%) |
| Enoxaparin during procedure | NA | NA | NA | 262 (46%) | 11 (0.4%) | 1738 (48%) | NA | NA | NA | NA | NA | NA | 0 | 94 (8.5%) |
| GPI; bailout in Biv, planned in Hp + GPI | 34 (24%) | 94 (100%) | NA | NA | 238 (9%) | 2473 (97%) | 217 (7%) | 2902 (96%) | 129 (7%) | 1699 (94%) | 0 | 100 | 125 (11%) | 766 (69%) |
| Radial access | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 4 (0.4%) | 2 (0.2%) | 510 (48%) | 502 (46%) |
| Procedure type, N | ||||||||||||||
| Any stent | 127 (88%) | 84 (89%) | NA | NA | 2407 (93%) | 2349 (93%) | 2548 (85%) | 2578 (86%) | 1571 (96%) | 1553 (95%) | 822 (96%) | 822 (96%) | 868 (92%) | 865 (91%) |
| DES | NA | NA | NA | NA | 1547 (60%) | 1543 (61%) | NA | NA | NA | NA | 757 (88%) | 764 (89%) | 538 (57%) | 529 (56%) |
| Atherectomy | NA | NA | NA | NA | 14 (0.5%) | 18 (0.7%) | 114 (4%) | 114 (4%) | NA | NA | NA | NA | 304 (32%) | 298 (31%) |
| Balloon only | NA | NA | NA | NA | 76 (3%) | 73 (3%) | 237 (8%) | 209 (7%) | NA | NA | 36 (4%) | 37 (4%) | 48 (5%) | 42 (4%) |
| Affected vessel, N | ||||||||||||||
| Left main | 2 (1.4%) | 2 (2%) | NA | NA | 40 (2%) | 43 (2%) | 36 (1%) | 39 (1%) | 13 (0.7%) | 7 (0.4%) | 25 (3%) | 27 (3%) | 6 (0.6%) | 13 (1.4%) |
| LAD | 61 (42%) | 33 (35%) | NA | NA | 1131 (43%) | 1047 (41%) | 1264 (42%) | 1282 (43%) | 700 (39%) | 747 (42%) | 347 (40%) | 316 (37%) | 425 (45%) | 423 (45%) |
| Left Cx | 44 (30%) | 29 (31%) | NA | NA | 930 (36%) | 881 (35%) | 883 (29%) | 865 (29%) | 293 (16%) | 269 (15%) | 231 (27%) | 241 (28%) | 115 (12%) | 132 (14%) |
| RCA | 60 (42%) | 31 (33%) | NA | NA | 921 (35%) | 976 (38%) | 1115 (37%) | 1035 (34%) | 757 (42%) | 738 (41%) | 227 (26%) | 236 (27%) | 417 (44%) | 412 (44%) |
| Bypass graft | NA | NA | NA | NA | 183 (7%) | 183 (7%) | 174 (6%) | 185 (6%) | 18 (1%) | 17 (1%) | 28 (3%) | 39 (4.5%) | 4 (0.4%) | 10 (1%) |
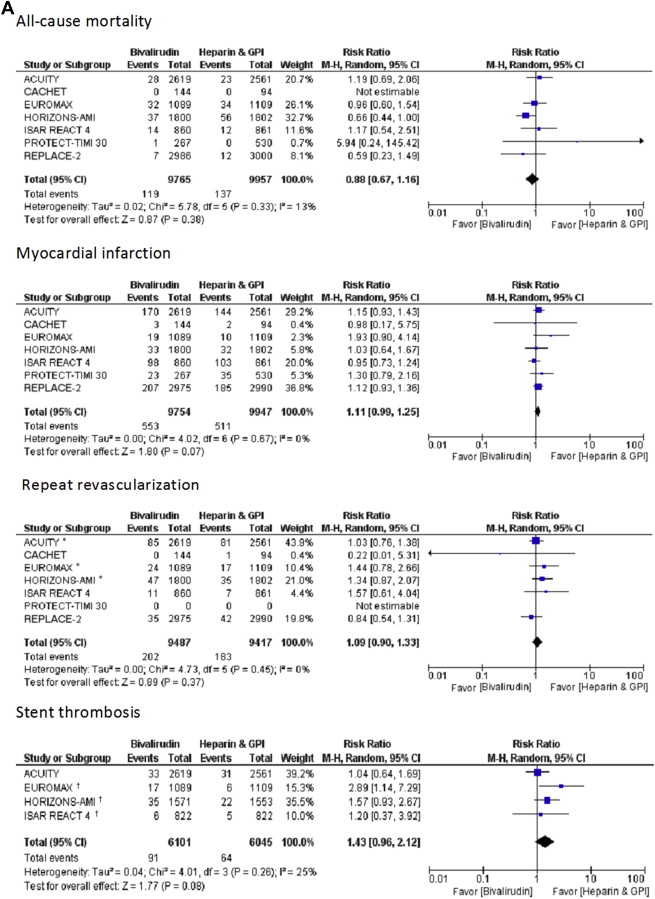
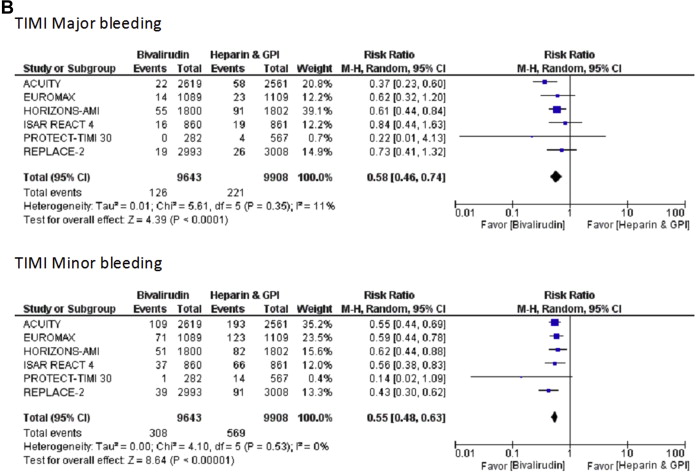
In patients undergoing PCI, bivalirudin monotherapy compared with heparin or enoxaparin plus GP IIb/IIIa inhibitors significantly decreased TIMI major bleeding (RR 0.58, 95% CI 0.46 to 0.74, p <0.0001) and TIMI minor bleeding rates (RR 0.55, 95% CI 0.48 to 0.63, p <0.0001), with similar rates of all-cause mortality (RR 0.88, 95% CI 0.67 to 1.16, p = 0.38) and repeat revascularization (RR 1.09, 95% CI 0.90 to 1.33, p = 0.37). However, bivalirudin use was associated with a trend toward more MIs (RR 1.11, 95% CI 0.99 to 1.25, p = 0.07) and ST at 30 days (RR 1.43, 95% CI 0.96 to 2.12, p = 0.08) compared with heparin and GP IIb/IIIa inhibitors. The trend of increased MIs and ST was driven primarily by the data from the 2 STEMI trials (HORIZONS-AMI and EUROMAX; Figure 2 ). When STEMI trials were excluded, the incidence of MI (RR 1.10, 95% CI 0.98 to 1.24, p = 0.12) and ST (RR 1.06, 95% CI 0.68 to 1.67, p = 0.79) at 30 days was similar between bivalirudin and heparin plus GP IIb/IIIa inhibitors groups ( Supplementary Figure 1 ). Bivalirudin use was also associated with less access site bleeding (RR 0.32, 95% CI 0.24 to 0.43, p <0.0001) and fewer blood transfusions (RR 0.60, 95% CI 0.47 to 0.77, p <0.0001) compared with heparin plus GP IIb/IIIa inhibitors strategy.
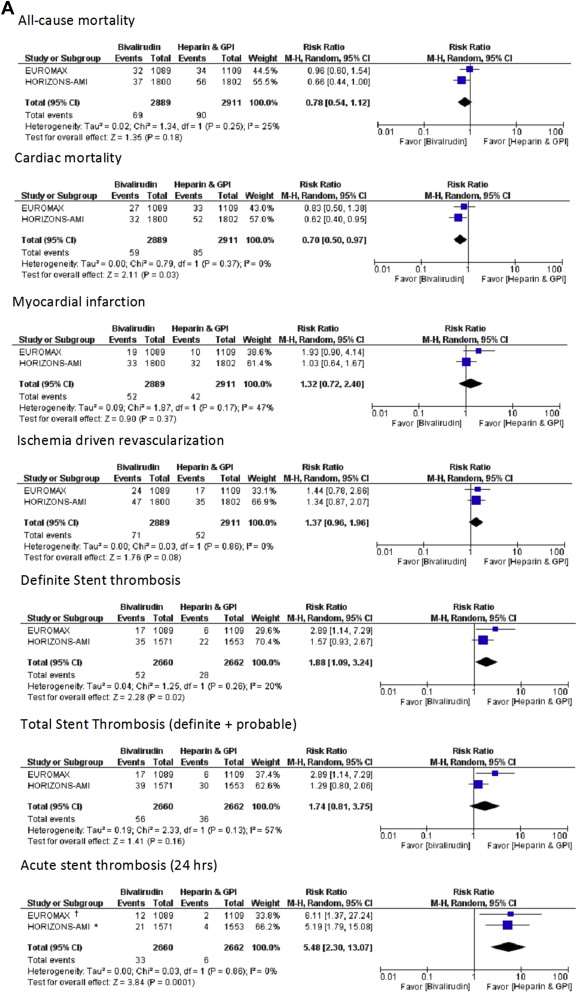
In patients with STEMI, bivalirudin use resulted in similar rates of all-cause mortality (RR 0.78, 95% CI 0.54 to 1.12, p = 0.18) and MI (RR 1.32, 95% CI 0.72 to 2.40, p = 0.37), a trend toward more repeat (ischemia-driven) revascularization (RR 1.37, 95% CI 0.96 to 1.96, p = 0.08), and, importantly, a reduction in cardiac mortality compared with heparin plus GP IIb/IIIa inhibitors (RR 0.70, 95% CI 0.50 to 0.97, p = 0.03; Figure 3 ). Similar to the overall PCI population, bivalirudin resulted in less TIMI major (RR 0.61, 95% CI 0.45 to 0.82, p = 0.0009) and TIMI minor bleeding (RR 0.60, 95% CI 0.48 to 0.75, p <0.0001; Figure 3 ). Bivalirudin use was also associated with fewer blood transfusions compared with heparin plus GP IIb/IIIa inhibitor use (RR 0.57, 95% CI 0.42 to 0.78, p = 0.0004). However, bivalirudin use was associated with more definite ST at 30 days (RR 1.88, 95% CI 1.09 to 3.24, p = 0.02), driven by an increase in acute ST (within 24 hours; RR 5.48, 95% CI 2.30 to 13.07, p = 0.0001). Total ST (definite plus probable) rates at 30 days were similar between 2 strategies (RR 1.74, 95% CI 0.81 to 3.75, p = 0.16; Figure 3 ), with similar subacute ST rates (RR 0.80, 95% CI 0.47 to 1.37, p = 0.42) in the 2 groups.