Abciximab is a glycoprotein IIb/IIIa receptor inhibitor that has been shown to improve outcomes in patients with ST-segment elevation myocardial infarction who undergo primary percutaneous coronary intervention (pPCI). An earlier study reported better efficacy with intracoronary (IC) compared to intravenous (IV) administration, but this finding has not been duplicated in other studies, thus leaving a great deal of uncertainty as to the most efficacious route of administration. To investigate if IC abciximab compared to IV administration decreases mortality and major adverse cardiac events in patients with ST-segment elevation myocardial infarction who undergo pPCI, a meta-analysis was performed consisting only of prospective randomized controlled trials. Subgroup analysis was performed to investigate the source of difference in efficacy between the 2 strategies. A meta-analysis of 4 trials including 1,148 subjects revealed that IC abciximab significantly reduced mortality compared to IV administration (1.5% vs 3.6%, odds ratio 0.44, 95% confidence interval 0.20 to 0.95, p = 0.04). Major adverse cardiac events were also reduced in a subgroup in which <30% of patients received aspiration thrombectomy (6.1% vs 16.2%, odds ratio 0.33, 95% confidence interval 0.18 to 0.61, p = 0.0004). In conclusion, the totality of the data available from relatively small but high-quality studies shows a significant mortality reduction associated using IC abciximab for pPCI compared to IV abciximab. IC abciximab in the setting of pPCI for ST-segment elevation myocardial infarction may be beneficial for patients with higher risk profiles.
Primary percutaneous coronary intervention (pPCI) is the preferred treatment for ST-segment elevation myocardial infarction (STEMI). The glycoprotein IIb/IIIa receptor inhibitor abciximab has been shown to be effective in reducing mortality and major adverse cardiac events (MACEs) in patients with STEMI who undergo pPCI. Experimental studies have suggested that abciximab exerts additional antiplatelet, antithrombotic, and anti-inflammatory effects when a higher local drug concentration is achieved by intracoronary (IC) administration. However, clinical studies on effects of IC abciximab have shown conflicting results, and a previous meta-analysis was significantly limited because of the heterogeneity of the studies. Recently, 2 additional randomized controlled trials of patients with STEMI were published, which presents the opportunity to reanalyze the totality of the data in a more thorough fashion. Therefore, the aim of this study was to compare the effects of IC versus intravenous (IV) abciximab administration on mortality and MACEs among patients with STEMI who underwent pPCI by performing a meta-analysis that consisted only of prospective randomized controlled trials.
Methods
We searched the MEDLINE, Embase, and Cochrane electronic databases through August 18, 2011. The key words and Medical Subject Headings used for the search were “abciximab,” “ReoPro,” “intracoronary,” “intravenous,” and “myocardial infarction.” Reports that included these terms were screened by their titles and abstracts for relevance. The search was performed without language or time limitations. References of relevant reports were also reviewed manually. Abstracts without following full-text publications, reviews, comments, letters, and works that were not original reports were excluded ( Figure 1 ).
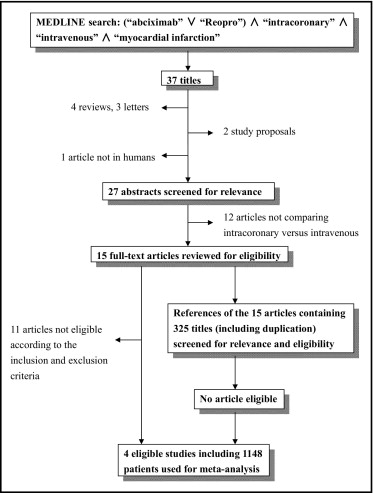
Studies were considered eligible if they (1) only included patients with STEMI, (2) randomly assigned patients to receive IC or IV boluses of abciximab, (3) blinded the investigators, and (4) prospectively followed mortality and MACEs for ≥30 days. Studies that met all the inclusion criteria but did not report clinical outcomes were also eligible as long as the corresponding author could provide the data. Studies that included patients with non–ST-segment elevation myocardial infarction or unstable angina pectoris were not eligible. Two investigators (Y.J.S. and Y.K.) independently retrieved the full text of the relevant reports to assess eligibility, and there was complete agreement between the 2 investigators on the final results.
Demographics of the study and patient, procedural, and angiographic characteristics were obtained ( Table 1 ). Numbers of deaths and MACEs (composite of death, nonfatal myocardial infarction, and repeat revascularization) in each treatment group were extracted from every study. If these data could not be obtained from the original report, the corresponding author was directly contacted to provide the data. The principal summary measures were odds ratios (ORs) for mortality and MACEs in the 2 treatment arms of each study.
| Iversen et al | Bertrand et al | Thiele et al | Gu et al | |||||
|---|---|---|---|---|---|---|---|---|
| IC | IV | IC | IV | IC | IV | IC | IV | |
| Variable | (n = 185) | (n = 170) | (n = 53) | (n = 52) | (n = 77) | (n = 77) | (n = 271) | (n = 263) |
| Study demographics | ||||||||
| End of recruitment period | 2008 | April 2008 | November 2008 | April 2010 | ||||
| Follow-up period | 30 days | 1 year | 30 days | 30 days | ||||
| Definition of MACEs | Death, recurrent MI, or TVR | Death, recurrent MI, and TVR | Death, reinfarction, urgent TVR, and occurrence of new CHF | Cardiac mortality, reinfarction, and TVR | ||||
| Patient characteristics | ||||||||
| Age (years) | 62 | 69 | 59 | 59 | 64 | 66 | 64 | 64 |
| Men | 82% | 80% | 77% | 83% | 82% | 77% | 77% | 71% |
| Hypertension | 40% | 40% | 43% | 44% | 70% | 74% | 44% | 49% |
| Dyslipidemia | 43% | 40% | 45% | 50% | 35% | 40% | 30% | 28 |
| Diabetes mellitus | 14% | 11% | 8% | 10% | 31% | 29% | 13% | 11% |
| Previous MI | 35% (prior CVD) | 30% (prior CVD) | 11% | 17% | 10% | 9% | 12% | 9% |
| Current smoking | 50% | 52% | 70% (current and past) | 77% (current and past) | 49% | 51% | 43% | 48% |
| Ischemic time (hours) | NR | NR | 3.0 | 3.0 | 4.1 | 3.6 | 3.0 | 3.0 |
| Procedural characteristics | ||||||||
| Clopidogrel loading dose (mg) | 300–600 | 300–600 | 600 | 600 | 600 | 600 | 600 (or prasugrel 60 mg) | |
| Aspiration thrombectomy | NR | NR | 40% | 44% | NR | NR | 98% | 97% |
| 12-hour intravenous infusion after bolus | 100% | 100% | 50% | 50% | 100% | 100% | 0% | 0% |
| Peak troponin (μg/L) | NR | NR | 3.93 | 4.37 | NR | NR | 3.03 | 4.36 |
| Left ventricular ejection fraction (%) | 38 | 40 | 64 | 64 | 48.0 | 46.1 | NR | NR |
| Angiographic characteristics | ||||||||
| Infarct-related vessel | ||||||||
| Left anterior descending artery | 48% | 51% | 42% | 40% | NR | NR | 45% | 47% |
| Left circumflex artery | 8% | 9% | 9% | 4% | NR | NR | 12% | 13% |
| Right coronary artery | 43% | 40% | 49% | 56% | NR | NR | 41% | 38% |
| Other | 1% | 0% | 0% | 0% | NR | NR | 2% | 2% |
| TIMI flow grade before PCI | ||||||||
| 0 | NR | NR | 57% | 60% | 57.1% | 67.5% | 46% | 55% |
| 1 | NR | NR | 11% | 15% | 11.7% | 6.5% | 9% | 12% |
| 2 | NR | NR | 26% | 17% | 7.8% | 5.2% | 24% | 19% |
| 3 | NR | NR | 6% | 8% | 23.4% | 20.8% | 21% | 14% |
| TIMI flow grade after PCI | ||||||||
| 0 | 1% | 2% | 2% | 0% | 1.3% | 2.6% | NR | NR |
| 1 | 4% | 7% | 0% | 0% | 1.3% | 1.3% | NR | NR |
| 2 | 14% | 18% | 6% | 10% | 13.0% | 10.4% | NR | NR |
| 3 | 81% | 73% | 93% | 90% | 84.4% | 85.7% | NR | NR |
| Number of Diseased Vessels | ||||||||
| 1 | 65% | 70% | NR | NR | 52% | 48% | 45% | 39% |
| 2 | 23% | 18% | NR | NR | 31% | 34% | 29% | 32% |
| 3 | 12% | 12% | NR | NR | 17% | 18% | 26% | 29% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


