This meta-analysis evaluated the optimal noninvasive strategy for cardiac risk assessment of patients >65 years of age with known or suspected coronary artery disease using the available literature. Patients >65 years of age constitute a growing proportion of the population and have higher cardiovascular morbidity and mortality, but an optimal strategy to predict the risk of cardiac events in this group is unknown. A systematic search of MEDLINE was performed for cohort studies of ≥100 patients >65 years old with ≥12 months of follow-up that reported cardiac death and/or nonfatal myocardial infarction after any of stress myocardial perfusion imaging (MPI), stress echocardiography, or exercise tolerance testing (ETT) for known or suspected coronary artery disease. Pooled annualized event rates were calculated for each technique. Summary odds ratios (ORs) between normal and abnormal test results were calculated using a random-effects model. Seventeen studies (MPI 7, stress echocardiography 7, ETT 3) in 13,304 patients (mean age 75.5 years) were included. Abnormal compared to normal stress MPI (OR 11.8, 95% confidence interval [CI] 7.5 to 18.7) and stress echocardiography (OR 3.2, 95% CI 2.6 to 3.9) accurately stratified risk in patients. However, patients with abnormal and normal ETT results had similar cardiac event rates (OR 3.1, 95% CI 0.8 to 11.5). In conclusion, stress imaging with MPI or stress echocardiography effectively stratified risk in patients, whereas ETT alone did not.
The number and proportion of Americans >65 years old are increasing. It is well known that the incidence of cardiovascular disease is higher in this group with approximately 80% of cardiovascular deaths occurring in patients >65 years old. Despite these findings, the optimal strategy to predict the risk of cardiac events in this group is unknown. To date, a comparison of noninvasive imaging strategies for risk stratification of this population is not available. We performed a meta-analysis of the published literature on the prognostic value of the 3 principal noninvasive techniques—stress myocardial perfusion imaging (MPI), stress echocardiography, and exercise tolerance testing (ETT)—using the standardized approach described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Methods
All methods for this review conformed with the PRISMA statement. We performed a systematic literature search of MEDLINE from its inception through August 2010 restricted to the English language. The search strategy combined the Medical Subject Heading term “prognosis” with Medical Subject Heading terms and keywords such as “single photon emission computed tomography (SPECT),” “exercise testing,” “stress echocardiography,” “thallium,” “technetium,” “dipyridamole,” “adenosine,+ and “dobutamine” for “aged” and “aged, 80 and over.” The study population was defined as >65 years of age. The Medical Subject Heading term “aged” included subjects 65 to 79 years and the term “aged, 80 and over” included subjects >80 years of age. A manual search of references from reports of prospective studies or review articles was performed to identify additional relevant articles. When applicable, efforts were made to contact investigators for clarification or additional data. Two independent reviewers assessed studies for inclusion in a parallel manner using criteria defined a priori as described in the study selection.
Articles were included if they (1) evaluated prognosis of patients with known or suspected coronary artery disease; (2) included patients with a mean age >65 years; (3) included ≥100 subjects; and (4) had a follow-up data of ≥12 months. Studies also were required to provide data on clinical outcomes of interest, including the composite of cardiac death and nonfatal myocardial infarction (MI) or cardiac death alone. Studies were excluded if they evaluated only preoperative risk stratification or populations after acute MI. Reports were eliminated if the meta-analysis investigators were unable to prepare 2 × 2 tables for clinical outcomes. For serial studies of a single or overlapping population, only the largest study was included.
Two investigators, using a standardized tool, independently abstracted all data, with disagreements resolved by a third investigator. The following information was sought from each published study: (1) author identification; (2) year of publication; (3) study design; (4) sample size; (5) inclusion/exclusion criteria; (6) duration of follow-up; (7) participant demographics; (8) type of stress study used (pharmacologic vs exercise); (9) radiopharmaceutical used for imaging; (10) type of imaging technique; and (11) number of clinical outcome events. Extracted demographic data included, but were not limited to, mean age, gender, and presence of cardiac risk factors such as hypertension, diabetes, hypercholesterolemia, known coronary artery disease, or previous MI. Data were recorded as normal or abnormal test results (nuclear perfusion, wall motion, ETT), and when available, abnormal results were classified further based on type of defect into scar versus ischemia. No studies reporting cardiac events after coronary computerized tomographic angiography in patients >65 years old were found. When data were incomplete for our analysis, individual study investigators were contacted. If data remained incomplete, event rates were estimated from Kaplan–Meier curves in the specific studies using the Engauge Digitizer 4.1 (available at: http://www.digitizer.sourceforge.net/ ). The time point used to extract event data from each study was estimated using average follow-up duration.
Quality analysis of all studies included in the meta-analysis was performed using a tool to evaluate the quality of prognostic studies previously described by Hayden et al. Potential sources of bias such as study participation and attrition rates, prognostic factor measurement, outcome measurement, confounding measurement, and rigor of analysis in each study were rated as “yes,” “partly,” “no,” or “unsure,” with a global score of “high” or “low” quality assigned to each study.
The primary analysis was the rate of cardiac events defined as cardiac death or MI. We performed a separate analysis of studies that reported the incidence of cardiac death alone. Event rates were pooled for each of the stress MPI, stress echocardiography, and ETT groups. A summary estimate of odds ratios (ORs) and associated 95% confidence intervals (CIs) was obtained using a DerSimonian–Laird random-effects model that used inverse-variance weighting and incorporated a component of variation between studies, assuming that they were a sample from a larger hypothetical population of studies. A comparison of annualized cardiac event rates (AERs) between each technique was also performed using inverse-variance weighting. AERs were calculated for each study as follows: total number of patients with events/([follow-up time {months}/12] × [total number of patients without events]). Statistical heterogeneity among studies was assessed using the I 2 statistic, with significant heterogeneity defined as I 2 <50%. Efforts were made to explore heterogeneity when necessary. Visual inspection of funnel plots and Egger weighted regression statistics were used to assess the presence of publication bias. All analyses were conducted using StatsDirect 2.7.7 (StatsDirect, Ltd., Cheshire, England) and Comprehensive Meta-Analysis 2 (Biostat, Englewood, New Jersey). A p value <0.05 was considered statistically significant.
An a priori subgroup analysis of studies reporting cardiac death was performed. After reviewing the initial data, several sensitivity analyses were undertaken to investigate potential confounders. Impact of pharmacologic stress protocols versus exercise stress was assessed by analyzing AERs in studies that used pharmacologic stress versus exercise stress with echocardiographic imaging or MPI. Because of the wide range of publication times of the included studies, sensitivity analysis was performed excluding studies that were published before 2000. Sensitivity analysis was also performed excluding studies that required extrapolation of data from printed Kaplan–Meier curves to investigate this technique.
Results
Figure 1 illustrates the stepwise search strategy for the literature review. After removal of duplicate citations, 7,227 articles were screened. Based on the title and abstract, 7,135 articles were excluded. The full text of the remaining 72 articles was evaluated for inclusion. After exclusions, 17 articles evaluating stress MPI, stress echocardiography, or ETT met the eligibility criteria. No reports of coronary computed tomographic angiography for risk stratification in a study population were available.
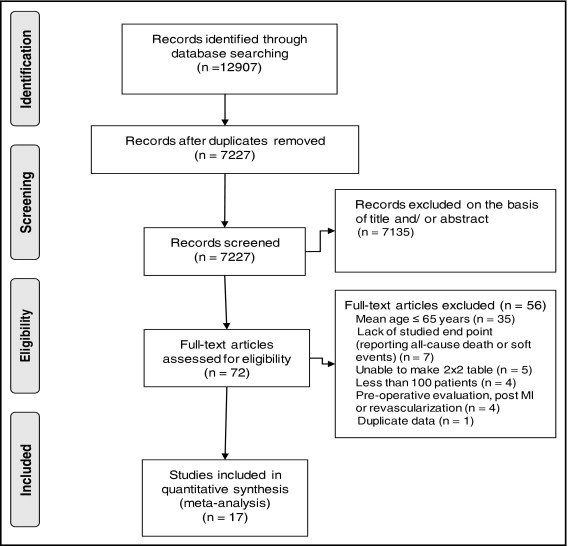
Table 1 presents 17 studies representing 13,304 patients (mean age 75.5 years) with a pooled mean follow-up time of 44.9 ± 23.0 months. Seven studies evaluating stress MPI in 1,585 patients met all criteria for inclusion. Two reported cardiac death only, 3 reported a composite of cardiac death and nonfatal MI, and 2 reported cardiac death and nonfatal MI separately. For stress echocardiography, 7 studies providing data on 9,131 patients were included; all reported a composite end point of cardiac death and nonfatal MI. Three studies evaluating ETT, with data on 2,588 patients, met the inclusion criteria. Of these, 1 reported composite cardiac events, 1 reported cardiac death only, and 1 reported cardiac death and nonfatal MI separately. With regard to the 3 pharmacologic stressors, 6 used dobutamine and 5 studies used dipyridamole as the stress agent. The 2 groups, exercise and pharmacologic stress, were comparable in radiotracers and imaging techniques used.
| Study | Pharm/Ex | Pharm Agent | Mean Age (years) | Number of Patients | Follow-Up (months) | AER After Negative Result (%) | AER After Positive Result (%) |
|---|---|---|---|---|---|---|---|
| Stress myocardial perfusion imaging | |||||||
| Shaw et al | Pharm | Dip | 75.0 ± 4.0 | 348 | 23 | 18.1 | 2.5 |
| Hilton et al | Ex | NA | 74.0 ± 4.0 | 120 | 36 | 6.0 | 0.5 |
| Lima et al | (49.1%) Pharm | Dip | 78.5 ± 3.4 | 167 | 34 | 11.4 | 1.1 |
| Zafrir et al | (71%) Pharm | Dip | 83.0 ± 3.0 | 162 | 45 | 9.7 | 0.7 |
| Schinkel et al | Pharm | Dob | 71.0 ± 5.0 | 247 | 39.6 | 9.5 | 1.7 |
| De Winter et al | (65%) Pharm | Dip | 78.0 ⁎ | 294 | 25.9 ⁎ | 5.9 | 2.2 |
| Valeti et al | Ex | NA | 77.6 | 247 | 76.8 | 5.4 | 0.9 |
| Stress echocardiography | |||||||
| Anthopoulos et al | Pharm | Dob | 75.3 ± 3.0 | 120 | 14 | 13.6 | 0.0 |
| Arruda et al | Ex | NA | 72.0 ± 5.0 | 2,632 | 34.8 | 2.9 | 0.7 |
| Biagini et al | Pharm | Dob | 73.0 ± 5.0 | 1,434 | 78 | 4.0 | 1.7 |
| Cortigiani et al | Pharm | Dip/Dob | 71 | 2,160 | 26 | 8.6 | 4.0 |
| Tsutsui et al | Pharm | Dob | 78.0 ± 5.0 | 399 | 21 | 10.8 | 1.9 |
| Innocenti et al | Pharm | Dob | 83.0 ± 3.0 | 227 | 36 | 8.3 | 2.4 |
| Bouzas-Mosquera et al | Ex | NA | 74.8 ± 3.4 | 2,159 | 42 | 6.9 | 2.7 |
| Exercise tolerance testing | |||||||
| Goraya et al | Ex | NA | 72.0 ± 6.0 | 514 | 75.6 | 5.1 | 1.7 |
| Kwok et al | Ex | NA | 77 | 247 | 84 | 3.4 | 2.4 |
| Lai et al | Ex | NA | 70.8 ± 4.6 | 1,827 | 72 | 3.0 | 1.7 |
Table 2 lists demographic characteristics within each study. Mean weighted averages of clinical cardiovascular risk factors were similar among the 3 testing techniques.
| Study | Men (%) | HTN (%) | DM (%) | Hyperlipidemia (%) | MI (%) | Smoking (%) |
|---|---|---|---|---|---|---|
| Stress myocardial perfusion imaging | ||||||
| Shaw et al | 50 | 57 | 23 | 20 | 29 | 16 |
| Hilton et al | 63 | NA | NA | NA | 26 | NA |
| Lima et al | 38 | 49 | 12 | 30 | NA | 5 |
| Zafrir et al | 62 | 41 | 12 | 27 | 33 | 7 |
| Schinkel et al | 52 | 41 | 19 | 30 | 36 | 21 |
| De Winter et al | 54 | 60 | 21 | NA | 38 | NA |
| Valeti et al | NA | 53 | 12 | 38 | NA | 36 |
| Weighted mean | 52% | 52% | 18% | 28% | 33% | 18% |
| Stress echocardiography | ||||||
| Anthopoulos et al | 60 | 65 | 23 | 66 | 40 | 40 |
| Arruda et al | NA | 51 | 11 | 55 | 24 | 7 |
| Biagini et al | 67 | 33 | 11 | 19 | 35 | 25 |
| Cortigiani et al | 57 | 50 | 18 | 36 | 42 | 25 |
| Tsutsui et al | 47 | 75 | 26 | 55 | 19 | 22 |
| Innocenti et al | 51 | 75 | 38 | 43 | 50 | 36 |
| Bouzas-Mosquera et al | 58 | 59 | 21 | 43 | 25 | 15 |
| Weighted mean | 59% | 51% | 16% | 42% | 31% | 18% |
| Exercise tolerance testing | ||||||
| Goraya et al | 52 | 43 | 10 | 54 | 22 | 10 |
| Kwok et al | 56 | 53 | 12 | 38 | 17 | 3 (current) |
| Lai et al | NA | NA | NA | NA | NA | NA |
| Weighted mean | 53% | 46% | 11% | 49% | 20% | 8% |
Results of quality analysis of the included studies are listed in Table 3 . Most studies adequately managed potential sources of bias, few studies demonstrated potential bias, and no studies signaled significant bias.
| Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Confounding Measurement and Account | Analysis | |
|---|---|---|---|---|---|---|
| Stress myocardial perfusion imaging | ||||||
| Shaw et al | + | + | + | + | partly | + |
| Hilton et al | partly | + | + | + | + | + |
| Lima et al | + | + | + | + | + | + |
| Zafrir et al | partly | + | partly | + | + | + |
| Schinkel et al | partly | + | + | + | + | + |
| De Winter et al | + | + | + | partly | + | + |
| Valeti et al | + | + | + | partly | + | + |
| Stress echocardiography | ||||||
| Anthopoulos et al | + | + | + | + | + | + |
| Arruda et al | + | + | + | + | + | + |
| Biagini et al | partly | + | + | + | + | + |
| Cortigiani et al | + | + | + | partly | + | + |
| Tsutsui et al | + | partly | + | + | + | + |
| Innocenti et al | partly | + | + | + | partly | + |
| Bouzas-Mosquera et al | + | + | + | + | + | + |
| Exercise tolerance testing | ||||||
| Goraya et al | + | + | + | + | + | + |
| Kwok et al | + | + | + | + | + | + |
| Lai et al | + | + | + | partly | + | + |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


