Loss-of-function (LOF) variants of cytochrome P450 2C19 (CYP2C19) have been hypothesized to be associated with lesser degrees of platelet inhibition and increased risk for recurrent ischemic events in patients with coronary artery disease on clopidogrel therapy; however, studies from Western countries have yielded mixed results. We aimed to assess the impact of CYP2C19 LOF variants on clinical outcomes from different ethnic groups. Sixteen prospective cohort studies including 7,035 patients carrying ≥1 CYP2C19 LOF allele and 13,750 patients with the wild-type genotype were included in this meta-analysis. Carriers of ≥1 CYP2C19 LOF allele were at significantly higher risk for adverse clinical events compared to noncarriers during clopidogrel therapy (odds ratio [OR] 1.42, 95% confidence interval [CI] 1.13 to 1.78). The summary OR showed a significant association between CYP2C19 LOF variants and an increased risk of cardiac death (OR 2.18, 95% CI 1.37 to 3.47), myocardial infarction (OR 1.42, 95% CI 1.12 to 1.81), and stent thrombosis (OR 2.41, 95% CI 1.76 to 3.30). Stratified analysis by ethnicity of study population suggested higher odds of adverse clinical events in the Asian population with LOF variants of CYP2C19 (OR 1.89, 95% CI 1.32 to 2.72) compared to Western populations (OR 1.28, 95% CI 1.00 to 1.64). In conclusion, carrier status for LOF CYP2C19 is associated with an increased risk of adverse clinical events in patients with coronary artery disease on clopidogrel therapy despite differences in clinical significance according to ethnicity.
Loss-of-function (LOF) polymorphisms associated with the cytochrome P450 2C19 (CYP2C19) gene have been associated with higher levels of platelet aggregation and are presumed to predict a substantial proportion of the variability in clinical response to clopidogrel. However, there has been no consensus on whether the CYP2C19 LOF polymorphism shows a similar impact according to ethnicity. Furthermore, there seem to be significant ethnic differences in the prevalence of the CYP2C19 polymorphism, with a higher frequency in East Asians and African-Americans than in Western populations. Previous studies and meta-analyses evaluating the influence of CYP2C19 polymorphism on clinical outcomes have been confined mostly to Western populations. We therefore conducted a systematic review and meta-analysis of eligible studies from different ethnic populations to determine the association of the LOF CYP2C19 polymorphism and cardiovascular risk in patients with coronary artery disease who use clopidogrel.
Methods
We identified relevant studies through electronic searches of MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and ISI Web of Science from 2001 through September 30, 2011. In addition, we manually searched the contents pages of issues published from 2010 through 2011 by the American College of Cardiology, the European Society of Cardiology, the Korean Society of Cardiology, the Transcatheter Cardiovascular Therapeutics, and the American Heart Association to retrieve further potential publications. Medical subject headings and keyword searches included the terms “clopidogrel,” “CYP2C19,” and “polymorphism.” If additional information was needed, the authors were contacted.
Two investigators (J.-S.J. and K.-I.C.) independently conducted the literature search, data extraction, and quality assessment using a standardized approach. Selected publications were reviewed by the same investigators to assess if studies met the inclusion criteria: (1) prospective cohort studies and randomized trials composed of patients with coronary artery disease who were treated with clopidogrel and (2) evaluation of CYP2C19 polymorphism and adverse clinical outcomes. Polymorphisms of CYP2C19 were designated by their National Center for Biotechnology Information Single Nucleotide Polymorphism database identifiers (“rs numbers”), nucleotide exchange, or common harmonized star allele nomenclature. Studies were excluded if the primary end point was only laboratory test results or if there was no appropriate comparison of outcomes. Two reviewers (J.-S.J. and B.-H.K.) independently extracted relevant information from the articles including patient characteristics, publication year, study period, ethnicity of study population, and data on specific outcomes.
The primary end point was the occurrence of adverse clinical events as defined in each study by the occurrence of death, nonfatal myocardial infarction (MI), stent thrombosis, or stroke. A secondary analysis was performed on mortality, which was defined as cardiovascular or overall mortality, MI, and stent thrombosis. End points were evaluated at the longest follow-up available. The definition of MI was different across studies, and we used the trial-specific definitions of MI. Stent thrombosis was definite or probable according to the definition of the Academic Research Consortium.
We used fixed-effects or random-effects models to produce across-study summary odds ratios (ORs) with 95% confidence intervals (CIs). Crude OR with 95% CI was used to assess the strength of association between CYP2C19 LOF allele and adverse clinical events in study populations. All p values were 2-tailed, with statistical significance set at 0.05. We assessed statistical heterogeneity between trials with the I 2 statistic, which is derived from the Cochran Q and the degree of freedom (100 × [{Q – degree of freedom}/Q]). I 2 values >25%, >50%, and >75% were considered evidence of low, moderate, and severe statistical heterogeneity, respectively. In case of heterogeneity across studies, we performed sensitivity analyses, serially excluding studies to determine the source of heterogeneity. In addition, sensitivity analyses were conducted to examine the heterogeneity by ethnicity in included patients to investigate possible differences between Western and Asian populations and the type of publication (abstracts only vs full reports). The likelihood of publication bias was assessed graphically by generating a funnel plot for adverse clinical outcomes and mathematically by the Egger test (p <0.1 for significant asymmetry). Correction for publication bias was performed using the trim-and-fill method. All statistical analyses were performed using Review Manager 5.1 (Nordic Cochrane Center, Copenhagen, Denmark) and MIX 2.0 (BiostatXL, Sunnyvale, California).
Results
Forty-eight publications were selected for inclusion and further evaluated. Subsequently, 16 studies were included in the final analysis ( Figure 1 ). Thirteen studies had been published in the peer-reviewed literature, whereas the other 3 studies were unpublished but presented at scientific meetings. Overall, 20,785 patients from 16 studies contributed to the adverse clinical events. In total 7,035 patients carrying CYP2C19 LOF alleles and 13,750 patients with the wild-type genotype were included in this meta-analysis. The main characteristics of the 16 studies are presented in Table 1 .
| Study | Year | Study Region | Ethnicity | Study Population | Number of LOF Alleles (2/1/0) | Clopidogrel Loading (mg) | Follow-Up Period | End Points | Publication Status | Result |
|---|---|---|---|---|---|---|---|---|---|---|
| EXCELSIOR | 2008 | Germany | Western | CAD with PCI | 17/228/552 | 600 | 12 mo | RPA; any death; nonfatal MI | published | higher RPA; no clinical difference |
| CLARITY-TIMI 28 | 2008 | USA and Europe | Western | STEMI | 4/73/150 | 300 | 30 d | occluded IRA; death; MI | unpublished | no difference |
| Malek et al | 2008 | Poland | Western | ACS with PCI | 1/20/84 | 300 or 600 | 12 mo | death or recurrent MI | published | no difference |
| TRITON-TIMI 38 | 2009 | USA and Europe | Western | ACS with planned PCI | 38/357/1,064 | 300 | 15 mo | CV death; MI; stroke | published | carriers worse |
| RECLOSE | 2009 | Italy | Western | CAD with PCI | 26/221/525 | 600 | 6 mo | definite or probable ST | published | carriers worse |
| CLEAR PLATELETS | 2009 | USA | Western | elective PCI | 5/63/160 | 300 or 600 | 12 mo | MI; stroke; ST, TVR, hospitalization; CV death | published | carriers worse |
| AFIJI | 2009 | France | Western | MI | 9/64/186 | — (≥1-mo use) | 6 mo | death; MI; urgent revascularization | published | carriers worse |
| FAST-MI | 2009 | France | Western | MI | 58/577/1,573 | 300∼600 | 12 mo | any death; nonfatal MI; stroke | published | no difference |
| ISAR | 2009 | Germany | Western | CAD with PCI | 47/633/1,805 | 600 | 30 d | definite ST | published | higher ST; no difference in combined end point |
| CURE | 2010 | Europe and Latin America | Western | NSTE-ACS | 61/589/1,880 | 300 | 3–12 mo | CV death; nonfatal MI; stroke | published | no difference |
| PLATO | 2010 | Europe and USA | Western | ACS | 125/1,263/3,516 | 600/300 | 12 mo | CV death; MI; stroke | published | no difference |
| Sawada et al | 2011 | Japan | Asian | — | 42 (1 or 2)/58 | 300 | — | death; MI; TVR; OCT-defined thrombus | published | no difference in MACE; higher thrombus |
| Tang et al | 2011 | China | Asian | CAD with PCI | 26/111/130 | — | 12 mo | angina recurrence; urgent revascularization; MI; ST; death | published | carriers worse |
| COACT | 2010 | Korea | Asian | angina, MI | 313/1,003/872 | — | 12 mo | any death; nonfatal MI; stroke | unpublished | no difference |
| Nisho et al | 2011 | Japan | Asian | — | 56 (1 or 2)/69 | — | — | death; MI; TLR | unpublished | no difference |
| SKY | 2012 | Korea | Asian | PCI with DES | 173/838/1,135 | 300 or 600 | 12 mo | CV death; nonfatal MI; ST | published | carriers worse |
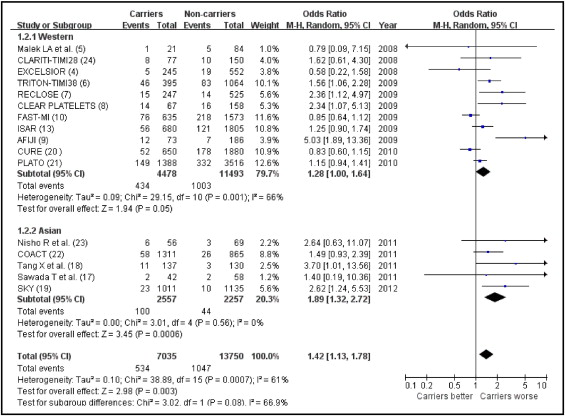
In total 1,581 of 20,785 patients developed the composite end point of death, MI, stent thrombosis, or ischemic stroke. Overall, a significantly increased risk of the primary end point was observed in carriers of ≥1 LOF allele of CYP2C19 compared to noncarriers (OR 1.42, 95% CI 1.13 to 1.78; Figure 2 ). Moderate statistical heterogeneity was noted among the included trials.
Sensitivity analysis of the risk of adverse clinical events in carriers of CYP2C19 LOF alleles after exclusion of each study yielded effect sizes similar in magnitude and direction to overall estimates. Nevertheless, there was evidence of significant heterogeneity for the primary end point across the included studies. After excluding 5 studies, significant heterogeneity was resolved (heterogeneity, chi-square 8.37, I 2 = 0%, p = 0.59) and the summary OR for carriers of CYP2C19 LOF allele was 1.60 (95% CI 1.33 to 1.93, p <0.001), which was not significantly different from the OR driven from analyzing all 16 studies.
Stratified and sensitivity analyses of risk of adverse clinical events limited to 5 Asian studies yielded effect sizes with the CYP2C19 LOF allele that were similar in direction and magnitude (OR 1.89, 95% CI 1.32 to 2.71), although different in statistical significance, with a more pronounced impact of genetic variants on clinical events in Asian populations ( Figure 2 ). Sensitivity analysis limited to 13 published studies yielded risk estimates similar in magnitude, direction, and significance to overall estimates (OR 1.39, 95% CI 1.07 to 1.80, p = 0.001).
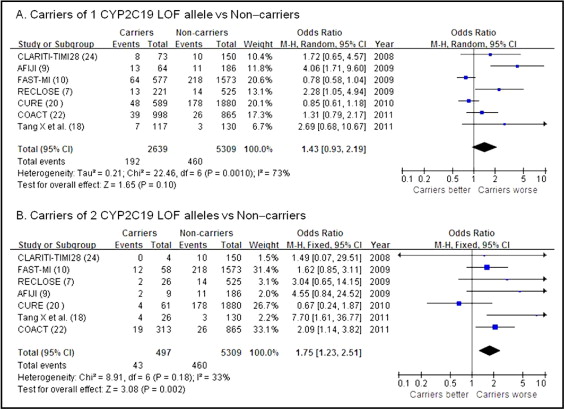
The relative contribution of 1 carrier or 2 carriers of CYP2C19 LOF alleles could be evaluated in 7 studies, representing 7,948 of 20,785 patients. The OR of adverse clinical outcomes for patients having 1 mutant allele was 1.43 (95% CI 0.93 to 2.19; Figure 3 ) with statistical heterogeneity, whereas the OR for subjects with 2 mutant alleles was 1.75 (95% CI 1.23 to 2.51; Figure 3 ) without significant heterogeneity.