Newer oral P2Y 12 inhibitors are more potent and have faster onset of action than clopidogrel. However, the efficacy and safety in patients with non–ST-elevation acute coronary syndrome (NSTE-ACS) are not well studied. A systemic search of MEDLINE and EMBASE databases was performed to identify randomized clinical trials comparing newer oral P2Y 12 inhibitors (prasugrel or ticagrelor) to clopidogrel in patients with NSTE-ACS. The primary outcome was a composite of cardiovascular death, myocardial infarction (MI), and stroke (major cardiovascular events [MACE]). Secondary outcomes were individual components of the primary outcome, all-cause mortality, and Thrombolysis In Myocardial Infarction (TIMI) major and minor bleeding. A total of 31,470 patients with NSTE-ACS from 4 randomized clinical trials were included (newer oral P2Y 12 inhibitors: 15,951; clopidogrel: 15,519). Newer oral P2Y 12 inhibitors significantly decreased MACE (relative risk [RR] 0.87, 95% confidence interval [CI] 0.80 to 0.95) and MI (RR 0.85, 95% CI 0.75 to 0.96) and showed a trend toward reduction of cardiovascular death (RR 0.89, 95% CI 0.71 to 1.01). There was a significant increase in TIMI major bleeding (RR 1.27, 95% CI 1.07 to 1.50) and TIMI major or minor bleeding (RR 1.20, 95% CI 1.02 to 1.42). Results were largely similar when stratified by ticagrelor versus prasugrel (p interaction >0.05) except for increased TIMI major/minor bleeding with prasugrel than ticagrelor (p interaction = 0.01). In conclusion, in patients with NSTE-ACS, newer oral P2Y 12 inhibitors decrease MACE and MI at the expense of a significant increase in the risk of bleeding. Treatment of 1,000 patients with newer oral P2Y 12 inhibitors will prevent 16 MACE and 13 MIs at the expense of increase in 6 major bleeding events.
Dual antiplatelet therapy comprising aspirin and clopidogrel is the cornerstone in management of patients with acute coronary syndromes (ACS) and in those who underwent percutaneous coronary intervention (PCI). Despite proved efficacy of clopidogrel in treatment of patients with ACS, its low bioavailability, relative slower onset of action, variability in patient responsiveness, and suboptimal platelet inhibition have led to the development of newer P2Y 12 inhibitors (prasugrel and ticagrelor). Prasugrel is an oral prodrug that irreversibly inhibits the P2Y 12 receptor, whereas ticagrelor is a direct-acting and a reversible inhibitor of the P2Y 12 receptor. Subsequently, several clinical trials have tested the efficacy and safety of prasugrel and ticagrelor compared with clopidogrel in patients with ACS. Non–ST-elevation acute coronary syndromes (NSTE-ACS) represent a spectrum of disease including unstable angina and non–ST-segment elevation myocardial infarction (NSTEMI). Among ACS, NSTE-ACS occurs more frequently than STEMI and is associated with worse long-term prognosis. However, none of the clinical trials involving newer oral P2Y 12 inhibitors were adequately powered to evaluate a difference in mortality compared with clopidogrel in patients with NSTE-ACS. Moreover, only 1 trial was adequately powered to detect difference in composite end point of cardiovascular death, myocardial infarction (MI), and stroke. Therefore, we undertook a systemic review and meta-analysis to investigate the impact of newer oral P2Y 12 inhibitors on the cardiovascular and bleeding events in patients with NSTE-ACS.
Methods
We performed a systematic search, without language restriction, using PUBMED, EMBASE, CINAHL, Web of Science, ClinicalTrials.gov , and Google Scholar from inception to February 2015 for randomized clinical trials (RCTs) comparing newer oral P2Y 12 inhibitors with clopidogrel in patients with NSTE-ACS. We also searched conference proceedings of the following societies: Transcatheter Cardiovascular Therapeutics, Euro-PCR, Society of Cardiovascular Angiography and Intervention, American College of Cardiology, American Heart Association, and European Society of Cardiology (ESC), presented in the last 10 years. Furthermore, we performed manual searches through the reference lists of studies, reviews, and pertinent meta-analysis on this topic. The search keywords included the following MeSH terms: (“prasugrel” OR “ticagrelor” OR “AZD6140” OR “P2Y 12 inhibitor” OR “Thienopyridine”) AND (“acute coronary syndrome” OR “myocardial infarction” OR “Non-ST-elevation myocardial infarction” OR “unstable angina” OR “Non-ST-elevation acute coronary syndrome”).
Studies were included if they met the following inclusion criteria: (1) RCTs comparing newer oral P2Y 12 inhibitors (prasugrel or ticagrelor) in patients with NSTE-ACS and (2) trials providing data on cardiovascular and/or bleeding outcomes. Exclusion criteria were the following (1) RCTs of intravenous P2Y 12 inhibitors (cangrelor or elinogrel) and (2) RCTs not including patients with NSTE-ACS or not reporting data for the NSTE-ACS cohort. Two reviewers (CB and SP) independently and in duplicate performed the literature search, reviewed the originally identified titles and abstracts, and selected studies for pooled analysis based on the inclusion and exclusion criteria. Any divergence was resolved with consensus. Quality of the included studies and assessment of trial bias risk was assessed for the domains recommended by the Cochrane collaboration, specifically emphasizing sequence generation, allocation concealment, blinding, outcomes assessment, and selective reporting.
The primary outcome was major adverse cardiovascular events (MACE) defined as a composite of cardiovascular death, MI, and stroke. We also evaluated individual components of the primary outcome, all-cause mortality, and major and minor bleeding. The definition of cardiovascular death, MI, and stroke followed the outcome definition of each study. Major bleeding was defined as Thrombolysis In Myocardial Infarction (TIMI) non–coronary artery bypass graft (CABG) major bleeding, except for 1 trial in which study protocol–defined major bleeding was used. For all the other studies, TIMI definitions of bleeding were considered for major or minor bleeding. Only 1 trial reported data on stent thrombosis; hence, we did not include this outcome in our analysis.
The statistical analysis was done in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines. Considering that the heterogeneity of the included trials might influence the treatment effects, we used the random-effects model to pool studies. The results were confirmed by a fixed-effects model to avoid small studies being overly weighted. Analysis was performed on an intention-to-treat basis. Heterogeneity was assessed using the Higgins’ and Thompson’s I-square statistics with values of <25%, 25% to 75%, and >75% corresponding to low, moderate, and high levels of heterogeneity. To compare the differential effect of individual P2Y 12 inhibitors (ticagrelor vs prasugrel), we performed stratified subgroup analysis by prasugrel and ticagrelor. Because only 1 trial reported results based on revascularization (PCI) strategy, we were unable to perform subgroup analyses stratified by PCI. However, we performed meta-regression analyses with the covariate of PCI use (% PCI) to assess the effect of PCI on outcomes. The other variables tested in the meta-regression analysis were age, percentage of men, hypertension, diabetes, history of MI, PCI, and CABG. These variables were selected based on the availability of data from all RCTs and clinical judgment. Publication bias was estimated visually by funnel plots and Egger’s regression test. A 2-tailed p <0.05 was considered statistically significant for all the analyses. Statistical analysis was performed using Stata 11 (Stata Corp., College Station, Texas) and RevMan, v5.02 (Nordic Cochrane Center, Copenhagen, Denmark).
Results
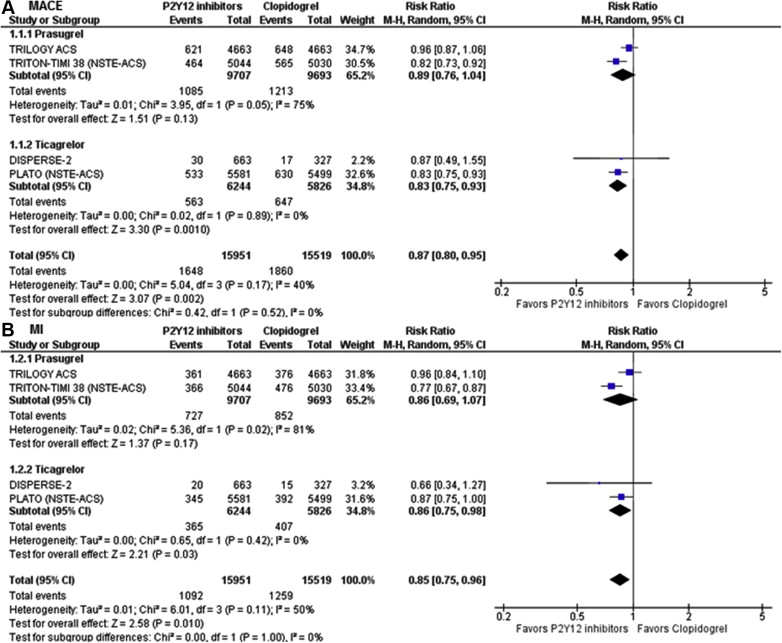
Our search identified 5 potential RCTs, of which 4 trials met our eligibility criteria ( Figure 1 ). We excluded 1 trial as it did not report outcomes separately in patients with NSTE-ACS. A total of 31,470 patients from 4 RCTs were included in the analysis. Of these, 15,951 patients were randomized to newer oral P2Y12 inhibitors, whereas 15,519 patients were randomized to clopidogrel. While Dose Confirmation Study Assessing Antiplatelet Effects of AZD6140 vs ClopidogRel in non–ST-Segment Elevation Myocardial Infarction (DISPERSE-2) and The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) were exclusively conducted in patients with NSTE-ACS, we used the data in the NSTE-ACS cohort of the Platelet Inhibition and Patient Outcomes (PLATO) trial and the Trial to Assess Improvement in Therapeutic outcomes by Optimizing Platelet Inhibition With Prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). The characteristics of the included trials are listed in Table 1 . All the included studies were of low bias risk. DISPERSE-2 and PLATO evaluated ticagrelor, whereas TRILOGY and TRITOM-TIMI 38 evaluated prasugrel ( Table 2 ).

| DISPERSE-2 | PLATO (NSTE-ACS) | TRILOGY ACS | TRITON-TIMI 38 (NSTE-ACS) | |
|---|---|---|---|---|
| Publication year | 2007 | 2014 | 2012 | 2014 |
| P2Y 12 inhibitor | Ticagrelor | Ticagrelor | Prasugrel | Prasugrel |
| Sample size | 984 | 11,080 | 9,326 | 10,074 |
| Maximal Follow-up | 12 weeks | 12 months | 30 months | 15 months |
| PCI | 42% | 52% | 0% | 99% |
| P2Y 12 Loading dose | None | 180 mg once | 30 mg once | 60 mg once |
| P2Y 12 Maintenance dose | 180 mg or 90 mg twice daily | 90 mg twice daily | 10 mg or 5 mg ∗ daily | 10 mg daily |
| Clopidogrel Loading dose | 300 mg or 600 mg once | 300 mg or 600 mg once | 300 mg once | 300 mg once |
| Clopidogrel Maintenance dose | 75 mg daily | 75 mg daily | 75 mg daily | 75 mg daily |
| Primary outcome | Total bleeding events | Composite of cardiovascular death, myocardial infarction, and stroke | Composite of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke | Composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke |
∗ In patients who were 75 years of age or older or who weighed less than 60 kg.
| Variables | DISPERSE-2 | PLATO (NSTE-ACS) | TRILOGY ACS | TRITON-TIMI 38 (NSTE-ACS) | ||||
|---|---|---|---|---|---|---|---|---|
| Ticagrelor (n=663) | Clopidogrel (n=327) | Ticagrelor (n=5,581) | Clopidogrel (n=5,499) | Prasugrel (n=4,663) | Clopidogrel (n=4,663) | Prasugrel (n=5,044) | Clopidogrel (n=5,030) | |
| Age (years) | 64±12 | 62±11 | 64 (56-72) ∗ | 64 (56-72) ∗ | 66(58-74) ∗ | 66(59-73) ∗ | 62±11 | 62±11 |
| Male | 63% | 66% | 69% | 68% | 61% | 61% | 74% | 72% |
| Hypertension | NA | NA | 70% | 70% | 82% | 82% | 69% | 69% |
| Hyperlipidemia | NA | NA | 52% | 52% | 59% | 59% | NA | NA |
| Diabetes | 24% | 25% | 29% | 28% | 38% | 38% | 25% | 24% |
| Smoker | NA | NA | 29% | 30% | 20% | 20% | NA | NA |
| Previous MI | 24% | 28% | 25% | 26% | 43% | 43% | 21% | 20% |
| Previous PCI | 14% | 17% | 17% | 17% | 26% | 27% | 16% | 16% |
| Previous CABG | 8% | 11% | 8% | 9% | 15% | 16% | 10% | 9% |
Newer oral P2Y 12 inhibitors significantly decreased MACE by 13% compared with clopidogrel (10.3% vs 12.0%; relative risk [RR] 0.87, 95% CI 0.80 to 0.95, p = 0.002). Similarly, newer oral P2Y 12 inhibitor reduced MI (6.8% vs 8.1%; RR 0.85, 95% CI 0.75 to 0.96, p = 0.01) with a trend towards reduction of cardiovascular death (3.8% vs 4.3%; RR 0.89, 95% CI 0.71 to 1.01, p = 0.07) compared with clopidogrel. All-cause mortality was numerically lower with newer oral P2Y12 inhibitors (5.7% vs 6.7%; RR 0.87, 95% CI 0.71 to 1.07, p = 0.19), although this was not statistically significant. There was no difference in stroke between the 2 groups (1.1% vs 1.2%; RR 0.96, 95% CI 0.78 to 1.18, p = 0.71; Table 3 ). The results were similar when stratified by prasugrel versus ticagrelor for MACE (p interaction = 0.52), MI (p interaction = 0.99), cardiovascular death (p interaction = 0.59), all-cause mortality (p interaction = 0.87), and stroke (p interaction = 0.98; Figures 2 and 3 ).
| Clinical Outcomes | P2Y 12 Inhibitor | Clopidogrel | RR (95% CI) | p value |
|---|---|---|---|---|
| MACE ∗ | 1,648/15,951 (10.3%) | 1,860/15,519 (12.0%) | 0.87 (0.80 -0.95) | 0.002 |
| All-cause mortality | 622/10,907 (5.7%) | 703/10,489 (6.7%) | 0.87 (0.71 -1.07) | 0.19 |
| Cardiovascular death | 604/15,951 (3.8%) | 673/15,519 (4.3%) | 0.89 (0.78-1.01) | 0.07 |
| Myocardial Infarction | 1,092/15,951 (6.8%) | 1,259/15,519 (8.1%) | 0.85 (0.75-0.96) | 0.01 |
| Stroke | 182/15,951 (1.1%) | 187/15,519 (1.2%) | 0.96 (0.78-1.18) | 0.71 |
| TIMI major bleeding † | 349/15,868 (2.2%) | 250/15,423 (1.6%) | 1.27 (1.07-1.50) | 0.007 |
| TIMI major and minor bleeding | 1,039/15,868 (6.5%) | 863/15,423 (5.6%) | 1.20 (1.02-1.42) | 0.03 |
∗ MACE included CV death/MI/Stroke.
† TIMI major bleeding included non-CABG TIMI major bleeding except in DISPERSE trial which included any major bleeding.