Aspirin is the most widely prescribed antiplatelet agent for the secondary prevention of stroke. Cilostazol, an antiplatelet and vasodilating agent, has shown promise for the secondary prevention of stroke. A systematic review and meta-analysis of randomized controlled trials using Ovid MEDLINE, PubMed, and Excerpta Medica (EMBASE) was searched up to October 2012. Four trials, in 3,917 patients, comparing cilostazol with aspirin were identified. Compared with aspirin, cilostazol was associated with a 73% reduction in hemorrhagic stroke (relative risk [RR] 0.27, 95% confidence interval [CI] 0.13 to 0.54, p = 0.0002), 28% reduction in the composite end point of stroke, myocardial infarction, or vascular death (RR 0.72, 95% CI 0.57 to 0.89, p = 0.003), and 48% reduction in total hemorrhagic events (RR 0.52, 95% CI 0.34 to 0.79, p = 0.002), with trend for lesser gastrointestinal bleeds (RR 0.60, 95% CI 0.34 to 1.06, p = 0.08). In conclusion, compared with aspirin, cilostazol is associated with significantly less hemorrhagic stroke, the combined end point of stroke, myocardial infarction, and vascular death, and total hemorrhagic events, with numerically fewer gastrointestinal bleeds when used for the secondary prevention of stroke.
Stroke causes approximately 10% of all deaths worldwide and is the sixth most common cause of reduced disability-adjusted life years. Although primary prevention is necessary for reducing the burden of stroke, secondary prevention is also extremely important. Approximately 30% of strokes occur in patients with a previous stroke or transient ischemic attack, with recurrent strokes being more severe and leading to greater consequences, such as dementia and death. Antiplatelet medications, such as aspirin, are routinely prescribed for the secondary prevention of stroke. However, meta-analyses indicate that aspirin is associated with only a 13% relative risk reduction for the secondary prevention of stroke. Moreover, the risk of hemorrhagic events limits the use of aspirin in this setting. Cilostazol, a phosphodiesterase III inhibitor, not only offers antiplatelet effects but also provides vasodilation, inhibition of vascular smooth muscle cell growth, and neuroprotection. We sought to perform a systematic review and meta-analysis of randomized controlled trials to determine if cilostazol reduces morbidity and mortality compared with aspirin for the secondary prevention of stroke.
Methods
A systematic review of the available published studies according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for the conduct of systematic reviews of intervention studies was performed.
Studies were identified through searches in the following sources: Ovid MEDLINE (2001 to 2012), PubMed (1982 to 2012), and EMBASE (2001 to 2012). To identify further potentially relevant studies missed by the electronic database search, reference lists from identified trials and reviews were manually screened. Searches were restricted to English language and updated using automated weekly email alerts until October 2012. Full details of the search strategies and excluded trials are available as appendices by request.
Studies were selected for inclusion on the basis of the following criteria: (1) study design: comparative randomized controlled trials, (2) type of participants: adults (≥18 years), (3) intervention: cilostazol, (4) comparator: aspirin, and (5) outcomes: all-cause mortality, cardiovascular death, ischemic stroke, hemorrhagic stroke, myocardial infarction (MI), hemorrhagic events, and gastrointestinal (GI) bleeds. The titles and abstracts of studies identified by the search strategy were independently screened by 3 reviewers (ARM, JJD, and HF), and clearly irrelevant studies were discarded.
The data elements extracted from each study were the number of patients per arm, the nature of the intervention, patient inclusion criteria, age, baseline and follow-up blood pressure, type of stroke severity and/or stroke subtype, and duration of follow-up (supplemental tables are available by request). The outcomes that were also extracted from each trial were all-cause mortality, cardiovascular death, ischemic stroke, hemorrhagic stroke, MI, hemorrhagic events, and GI bleeds. Quality assessment was judged according to the following criteria: concealment of treatment allocation; similarity of both groups at baseline regarding prognostic factors and medication use; blinding of outcome assessors, care providers, and patients; completeness of follow-up; and intention-to-treat analysis (supplemental tables are available by request). The overall study quality was quantified using the Jadad score. The data extraction and quality assessment was undertaken using standardized pro forma by 2 reviewers (HF and ARM).
We expressed outcome results for each study as relative risk (RR; 95% confidence interval [CI]). Summary estimates were computed using a DerSimonian and Laird random effects meta-analysis model. We report pooled results as RR and number needed to treat (NNT). Statistical heterogeneity across trials was estimated using the I 2 statistic, in which I 2 <30% denotes “low heterogeneity,” I 2 = 30% to 50% represents “moderate heterogeneity,” and I 2 >50% denotes “substantial heterogeneity.” A 2-tailed p value <0.05 was considered as statistically significant for all analyses. Cochrane Review Manager (RevMan v.5) software was used for all analyses.
Results
The search in published studies yielded 367 titles, of which 5 were reviewed in full text on the basis of the inclusion criteria ( Figure 1 ). Of these, 4 studies were deemed eligible for inclusion ( Figure 1 ). Tables containing the characteristics of the included studies are available by request.
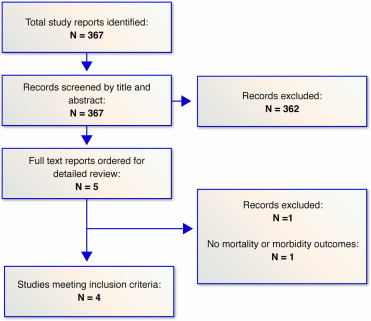
All trials were comparison randomized controlled trials of cilostazol compared with aspirin for the secondary prevention of stroke (none were comparing dual antiplatelet therapy). All background medications and/or baseline characteristics were statistically similar between the comparison groups in each trial, except for the Cilostazol Stroke Prevention Study 2 trial, which had significantly more patients in the aspirin group on antihypertensive medications (p <0.0001), including angiotensin II receptor blockers (p <0.0001) and calcium channel blockers (p <0.001), both of which are known to reduce the risk of stroke. Trials enrolled a mean of 979 patients with a mean follow-up of 15 months.
All 4 studies scored well on the methodologic quality indicators (supplemental tables with details are available by request). Concealed allocation and blinding of ≥1 outcome assessment was stated in all 4 trials.
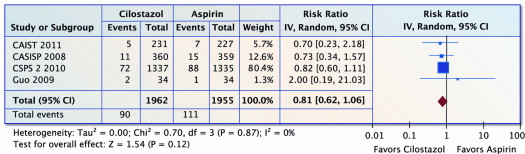
Four trials (n = 3,917) reported on ischemic stroke. There was a nonsignificant 19% reduction in ischemic stroke with cilostazol compared with aspirin (RR 0.81, 95% CI 0.62 to 1.06, p = 0.12 and I 2 = 0%; Figure 2 ).
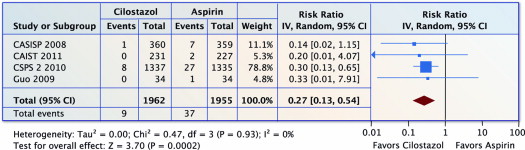
Four trials (n = 3,917) reported on hemorrhagic stroke. Compared with aspirin, cilostazol was associated with a 73% reduction in hemorrhagic stroke (RR 0.27, 95% CI 0.13 to 0.54, p = 0.0002 and I 2 = 0%; Figure 3 ). The NNT was 70 (95% CI 46 to 129).
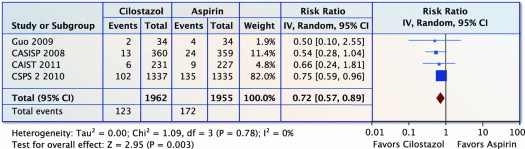
Four trials (n = 3,917) reported on stroke, MI, or vascular death. Compared with aspirin, cilostazol was associated with a 28% reduction in stroke, MI, or vascular death (RR 0.72, 95% CI 0.57 to 0.89, p = 0.003 and I 2 = 0%; Figure 4 ). The NNT was 40 (95% CI 24 to 114).
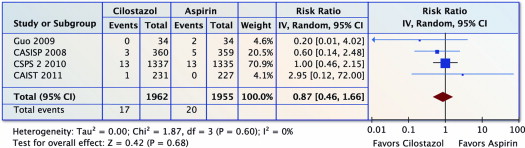
Four trials (n = 3,917) reported on all-cause mortality. There was no difference in all-cause mortality with cilostazol versus aspirin (RR 0.87, 95% CI 0.46 to 1.66, p = 0.68 and I 2 = 0%; Figure 5 ).
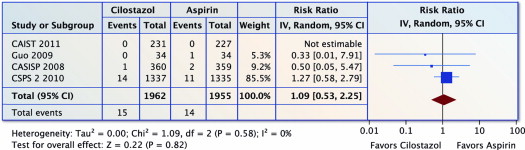
Four trials (n = 3,917) reported on MI. There was no difference in MI with cilostazol versus aspirin (RR 1.09, 95% CI 0.53 to 2.25, p = 0.82 and I 2 = 0%; Figure 6 ).
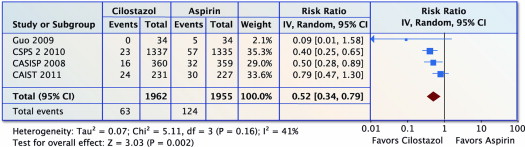
Four trials (n = 3,917) reported on total hemorrhagic events. Compared with aspirin, cilostazol was associated with a 48% fewer total hemorrhagic events (RR 0.52, 95% CI 0.34 to 0.79, p = 0.002 and I 2 = 0%; Figure 7 ). The NNT was 32 (95% CI 22 to 55).