Dronedarone is a benzofuran derivative approved by the Food and Drug Administration to decrease the risk of cardiovascular hospitalization in patients with paroxysmal or persistent atrial fibrillation (AF) and associated cardiovascular risk factors who are in sinus rhythm or will undergo cardioversion. There has been recent evidence to suggest that dronedarone may not have a favorable safety profile. We decided to evaluate all available evidence on the cardiovascular safety of this drug. A systematic search was made of the PubMed, CENTRAL, and EMBASE databases for randomized controlled trials from 1966 through 2011 comparing dronedarone to comparators in AF/heart failure. Intervention was dronedarone for AF for some studies and heart failure for others. Comparators included standard medical therapy and/or placebo and amiodarone for 1 study. Outcomes assessed were all-cause mortality, cardiovascular mortality, ventricular arrhythmias, embolic events, acute coronary syndrome, heart failure exacerbations, and hospitalization rates in the intervention versus comparator group at the end of ≥3 months of follow up with abstraction of data by 1 author. Seven randomized controlled trials were included in our analysis. Dronedarone use was associated with a trend toward worse all-cause and cardiovascular mortalities and increased heart failure exacerbations. It also showed numerically higher event rates for all other outcome events except acute coronary syndrome. Our pooled analysis showed increased all-cause and cardiovascular mortalities and increased heart failure exacerbations with use of dronedarone across a wide spectrum of populations. In conclusion, we recommend exercising caution using dronedarone, especially in patients with cardiovascular risk factors.
Dronedarone is a novel noniodinated benzofuran derivative with class I, II, III, and IV antiarrhythmic properties and an electropharmacologic profile closely resembling that of amiodarone but with structural differences minimizing the adverse effects of amiodarone on thyroid and pulmonary functions. In addition, the elimination 1/2 life of dronedarone is much shorter than for amiodarone. In a short-term study, dronedarone was less effective than amiodarone in decreasing atrial fibrillation (AF) recurrence but had a better safety profile. Some clinical trials have shown that dronedarone 400 mg 2 times/day is effective in the prevention of AF relapses and long-term maintenance of sinus rhythm. The Food and Drug Administration has approved dronedarone use for preventing rehospitalizations in patients in sinus rhythm and with cardiovascular risk factors (i.e., >70 years old, hypertension, diabetes, previous cerebrovascular accident, left atrial diameter ≥50 mm, or left ventricular ejection fraction <40%). In A Placebo-Controlled, Double-Blind, Parallel-Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter (ATHENA), dronedarone decreased the incidence of the primary outcome of unplanned hospitalization for cardiovascular causes or death. Significant decreases in rates of death from cardiovascular causes and stroke were also seen. However, dronedarone increased rates of stroke, heart failure, and death from cardiovascular causes in patients with permanent AF and cardiovascular risk factors in the recent Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS), prompting the Food and Drug Administration to undertake a safety review focusing particularly on the cardiovascular safety of dronedarone. In this review, we systematically evaluate the cardiovascular safety profile of dronedarone across the spectrum of patient populations in which it has been tested.
Methods
We systematically searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials for trials that randomized study participants to dronedarone versus a comparator from 1966 through December 2010. The following medical subject heading terms were included for a MEDLINE search and adapted for other databases as needed: “dronedarone,” “multaq,” “SR 33589,” “atrial fibrillation,” and “atrial flutter.” In addition to searching databases, reference lists of all included studies, meta-analyses, and reviews were manually searched. There was no language restriction for the search.
We included trials that studied adult (≥18-year-old) patients and reported relevant clinical outcomes with dronedarone. Eligible trials had to be randomized clinical trials comparing dronedarone to comparators, have randomized >100 patients to have adequate power, and have reported the outcomes of interest. We included all trials that randomized dronedarone because our objective was to study cardiovascular outcomes with dronedarone use in a wide variety of populations. We excluded trials of patients where dronedarone was used as an adjunct, those in which dronedarone was also used in the control group, and those that did not report the outcomes of interest.
S.C. reviewed the trials to ensure that they met the inclusion criteria and abstracted the data, and these were checked for accuracy by the other authors. Disagreements were resolved by consensus (10% of the time). We performed objective assessment of trials using the method specified in the Cochrane Handbook of Systematic Reviews assessing for randomization, concealment, blinding, intention to treat, baseline comparisons, concomitant interventions, and completeness of follow-up.
Our primary outcome was all-cause mortality and cardiovascular mortality at the end of the follow-up period for the trial. Maximum duration of follow-up assessed for the study was 26 months. Secondary outcomes of interest were ventricular tachyarrhythmias, stroke and systemic embolism, acute coronary syndrome (encompassing unstable angina and myocardial infarction), heart failure exacerbations (and hospitalizations), and rehospitalizations. We also performed exploratory subanalyses to identify and delineate cardiovascular effects in different subgroups of patients.
Meta-analysis was performed according to recommendations of the Cochrane Collaboration and in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Pooled treatment effects were estimated using risk ratio (RR) with the Mantel–Haenszel risk ratio in a random-effects model. Heterogeneity was assessed by chi-square tests and I 2 statistic; we defined I 2 <25% as low heterogeneity according to the Cochrane Handbook of Systematic Reviews. Publication bias was estimated using funnel plots and Egger regression test.
We performed a sensitivity analysis to explore the effects of identifying and excluding the study that contributed to the heterogeneity of the results and performed exploratory analyses to eliminate bias from studies that looked at the effects of dronedarone in patients with heart failure and those with permanent AF. We assessed quality for each included trial; all included trials were randomized controlled trials and were considered high quality. For statistical analysis we used Review Manager 5.1 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) and STATA 11 (STATA Corp., College Station, Texas).
Results
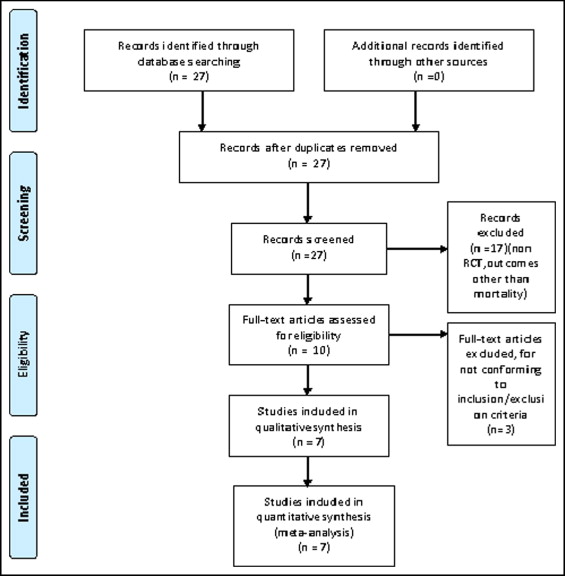
Our MEDLINE search yielded 27 studies. After elimination of duplicate results, the EMBASE and Cochrane registries did not yield additional studies. Through a review of titles and abstracts, 17 studies were rejected for lack of relevance. The remaining 10 articles were reviewed and assessed for satisfaction of the inclusion or exclusion criteria. The 7 articles that met all criteria were included in this analysis ( Figure 1 ).
Trials of paroxysmal and persistent AF were fairly homogenous with respect to inclusion and exclusion criteria, with a few key differences ( Tables 1 and 2 ). We also included 1 trial that randomized patients with heart failure and another trial that looked at permanent AF. Most studies excluded subjects with high-degree atrioventricular blocks, bradycardia, prolonged corrected QT interval, and an implantable cardioverter–defibrillator. Dronedarone was used in most studies (except the Dronedarone Atrial Fibrillation Study after Electrical Cardioversion [DAFNE] ) at an approved dose of 400 mg 2 times/day. All studies reported outcomes ranging from 3 to 26 months. Only 1 study included an active comparator and compared dronedarone to amiodarone.
| Trial Name | Dronedarone (mg) | Comparator | Inclusion Criteria | Exclusion Criteria | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | AF | HF | AF | HF | Heart Block | Increased QTc | |||
| ADONIS-EURIDIS 2007 | 400 2 times/day | placebo | 21 | paroxysmal | — | permanent | + | + | + |
| ANDROMEDA 2008 | 400 2 times/day | placebo | 18 | 0 | + | — | — | + | + |
| ATHENA 2009 | 400 2 times/day | placebo | 70 | paroxysmal | 0 | + | + | + | — |
| DAFNE | 400, 800, 1,600/day or 400, 600, 800 2 times/day | placebo | 21–85 | persistent | — | 0 | 0 | 0 | 0 |
| DIONYSOS 2010 | 400 2 times/day | amiodarone 600 mg/day, then 200 mg/day | 21 | persistent | — | paroxysmal | + | + | + |
| ERATO 2008 | 400 2 times/day | placebo | 21 | permanent | — | — | 0 | 0 | 0 |
| PALLAS 2011 | 400 2 times/day | placebo | 65 | permanent | + | paroxysmal | — | 0 | 0 |
| Trial | Mean Age (years) | Men | Duration of Follow-Up (months) | Total Number of Patients | ||
|---|---|---|---|---|---|---|
| Dronedarone Arm | Placebo Arm | Dronedarone Arm | Placebo Arm | |||
| ADONIS-EURIDIS 2007 | 63.5 ± 10 | 62 ± 11 | 68.5% | 70% | 12 | 1,237 |
| ANDROMEDA | 71 | 72 | 74% | 76% | 6 | 627 |
| ATHENA | 71 ± 9 | 71 ± 9 | 51% | 55% | 21 ± 5 | 4,628 |
| DAFNE | 63 | 65 | 65% | 79% | 6 | 270 |
| DIONYSOS 2010 | 64 ± 10 | 64 ± 10 | 70% | 71% | 6 | 504 |
| ERATO 2008 | 65 | 66 | 68% | 70% | 6 | 174 |
| PALLAS 2011 | 75 ± 6 | 75 ± 6 | 65% | 64% | 3.5 | 3,236 |
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses using Cochrane metrics to evaluate the quality of studies selected for this review.
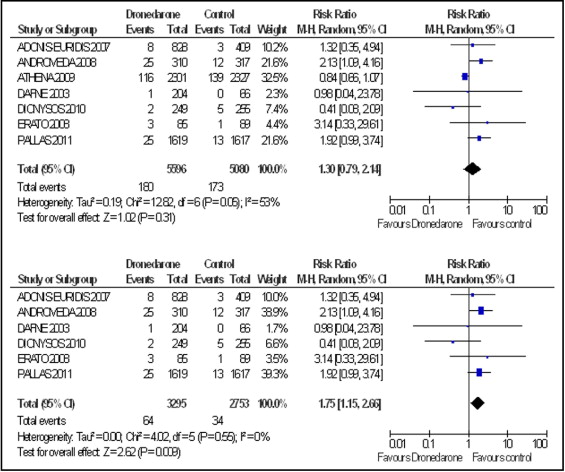
We identified 7 articles, accounting for 10,676 subjects, that met our inclusion and exclusion criteria ( Figure 1 ). Dronedarone use was associated with a trend toward worse all-cause mortality (p = 0.31; Figure 2 ) and cardiovascular mortality (p = 0.28; Figure 3 ), with significant heterogeneity in the outcomes. We identified ATHENA to be the source of the heterogeneity and, hence, performed our analysis including and then excluding the data from ATHENA. When data from ATHENA was excluded, dronedarone use was associated with worse all-cause mortality (p = 0.009) and cardiovascular mortality (p = 0.0002), without any heterogeneity. Also, publication bias was assessed by constructing a funnel plot and Egger test with a 2-tailed p value (p = 0.14).