Mesenteric Arteries
Aaron C. Baker
Evaluation of the mesenteric arteries is an important duplex application because it allows the noninvasive vascular laboratory to diagnose chronic mesenteric ischemia, a life-threatening disorder. Mesenteric duplex scanning can help prevent severe morbidity and mortality in these patients who often go undiagnosed for prolonged periods of time. The diagnosis of chronic mesenteric ischemia is difficult because the natural history is not well understood and the symptoms are often nonspecific. Prior to the validation of duplex ultrasound for evaluating the mesenteric arteries, the only way to confirm mesenteric artery stenosis in a patient with the clinical suspicion for chronic mesenteric ischemia was contrast arteriography. Nicholls et al from the University of Washington were among the first to report the use of ultrasound for the diagnosis of mesenteric artery disease in 1986.1 These investigators described the hemodynamic characteristics of normal and abnormal mesenteric arteries based on Doppler velocity waveforms. In 1991, two large retrospective studies identified duplex flow velocities that allowed accurate identification of severe mesenteric artery stenosis.2,3 Since then, the mesenteric duplex examination has been widely adopted, and several prospective studies have established the accuracy of the velocity thresholds.4,5 Like renal duplex scanning (see Chapter 24), the mesenteric duplex examination entails a substantial learning curve for both the sonographer and the interpreting physician. However, when mastered, this is a valuable and rewarding study.
The goals of intervention for chronic mesenteric ischemia are to alleviate symptoms, restore normal body weight, and prevent bowel infarction. The number of mesenteric revascularizations has increased substantially over the last decade, largely because of improved diagnosis and the decreased morbidity associated with endovascular therapy. In most centers, mesenteric angioplasty and stenting is the first choice of treatment over open surgical revascularization in patients with chronic mesenteric ischemia who have suitable lesions.6,7,8 While there are no prospective randomized comparisons between the two therapeutic approaches, retrospective reviews show decreased 30-day mortality, decreased perioperative morbidity, and shorter length of stay and convalescence time with endovascular revascularization compared to open surgery. However, open mesenteric bypass offers better patency, lower rates of reintervention, and improved freedom from recurrent symptoms.6,8,9,10,11,12,13,14,15,16,17,18
INDICATIONS FOR MESENTERIC ARTERY SCANNING
Mesenteric duplex scanning is an excellent screening test for patients with symptoms of chronic mesenteric ischemia. The most common cause of chronic mesenteric ischemia is atherosclerotic disease, and the majority of patients will have a history of smoking, hypertension, and hyperlipidemia; however, nonatherosclerotic conditions including vasculitis, dissection, fibromuscular dysplasia, radiation arteritis, mesenteric venous occlusion, drug-induced arteriopathy, and midaortic syndrome can present with a similar clinical picture. Classic signs and symptoms include abdominal pain, weight loss, and “food fear.” Typically, the pain is mid abdominal and dull or crampy, and it begins within 30 minutes after meals and can last for as long as six hours. Some patients learn to avoid certain foods or complain of nausea, vomiting, or a change in bowel habits, all without postprandial onset.
Although it may be argued that patients with the textbook presentation of postprandial pain, weight loss, and “food fear” should proceed directly to diagnostic angiography, duplex scanning is an appropriate starting point for patients with less classic presentations. Most patients with symptomatic chronic mesenteric ischemia are found to have severe stenosis or occlusion in multiple mesenteric arteries. The role of duplex scanning in the evaluation of suspected acute mesenteric ischemia is much more limited because those patients are usually critically ill and are difficult to examine with ultrasound.
Mesenteric duplex scanning is being used for follow-up after endovascular interventions, including superior mesenteric artery (SMA) and celiac artery stents.19,20,21,22 Given that these stents are prone to restenosis, duplex scanning is an ideal surveillance method. While a few retrospective studies have shown that the velocity thresholds used to diagnose native mesenteric artery stenosis overestimate the severity of stenosis in stented mesenteric arteries, there are currently no standard duplex criteria for the detection of mesenteric in-stent restenosis.19,20,21
Duplex ultrasound has also been used in diagnosing median arcuate ligament syndrome. While the pathophysiology and clinical significance of median arcuate ligament syndrome are controversial, mesenteric duplex can be a useful adjunct in determining the presence of this condition. An elevated peak systolic velocity (PSV) in the celiac artery during expiration, which normalizes with deep inspiration, is typically found; however, this respiratory variation can be a normal physiologic finding in healthy, asymptomatic patients.23 There are currently no duplex ultrasound findings that have been validated for the diagnosis of median arcuate compression of the visceral arteries. Ultimately, the diagnosis and decision to intervene are made in combination with the clinical history, physical examination, and additional imaging studies. Finally, duplex scanning may be used for the evaluation of portal, mesenteric, and hepatic venous disorders (see Chapter 22).
DUPLEX TECHNIQUE
It is important to perform the mesenteric duplex examination after the patient has fasted overnight, or for at least 4 hours prior to the examination, because the SMA velocity waveform changes from a low-flow, high-resistance pattern to a high-flow, low-resistance pattern after eating. Velocity thresholds for identifying significant stenosis of the celiac artery and SMA have been established for patients in the fasting state, so application of these standard criteria requires that the examination be performed in fasting patients. Typically, the patient should be instructed not to eat or drink anything starting at midnight and schedule the duplex scan early the next day to minimize abdominal gas. Regular medications can be taken with a few sips of water. Diabetic patients are instructed to consult with their primary care provider to alter insulin or other medications appropriately, and all patients are asked to refrain from smoking or chewing gum on the morning of the examination.
The mesenteric duplex examination is performed with the patient supine. A slight reverse Trendelenburg (feet down) position or head elevation may be helpful. Alternatively, a lateral decubitus position may be used in patients with large abdominal girth or when bowel gas interference is encountered with the patient supine. Low-frequency transducers are required, with the exact frequency range determined by the body habitus of the patient.
The scan head is initially placed just below the xiphoid process and slightly to the left of the midline for identification of the most proximal abdominal aorta. B-mode is used initially, scanning transversely through the length of the abdominal aorta for determining maximum size and presence of plaque to the level of the bifurcation. The aorta, celiac artery, common hepatic artery, and SMA should be interrogated thoroughly by pulsed Doppler, and spectral waveforms should be recorded from these vessels. This will require a combination of sagittal and transverse views. The Doppler sample volume should be “walked” from the aorta into the origins of the celiac artery and the SMA to identify the highest PSV and end-diastolic velocity (EDV). Attempts should be made to identify and assess the inferior mesenteric artery (IMA), particularly if significant occlusive disease is found in the celiac artery or SMA.
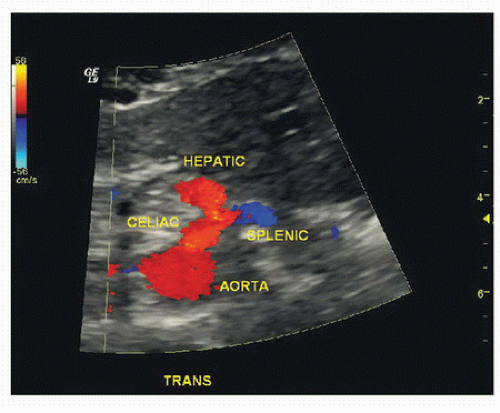
The celiac artery arises from the anterior aspect of the aorta 1 to 2 cm below the diaphragm and is usually best imaged in a transverse plane, showing its origin and bifurcation into the common hepatic artery (going toward the liver on the patient’s right) and the splenic artery (going toward the spleen on the patient’s left). This view is often referred to as the “seagull sign” and allows for accurate flow velocity measurements at the origin of the celiac artery utilizing a relatively small or zero degree Doppler angle (Fig. 23.1). The normal Doppler waveform from the celiac artery has a low-resistance pattern with antegrade flow continuing throughout systole and diastole. This Doppler waveform morphology occurs because the celiac artery supplies two low-resistance solid organs—the liver and spleen (Fig. 23.2).
Careful attention to Doppler angle correction for velocity measurements is necessary because these vessels may be tortuous. Close attention should also be given to the Doppler pulse repetition frequency (PRF) and the orientation of the color-scale bar. The colors on the top half of the scale are assigned to flow coming toward the scan head, and the colors on the bottom half represent flow away from the scan head. Flow direction becomes especially important when the celiac artery is occluded or severely stenotic. In this situation, low pressure in the celiac artery induces SMA collaterals to divert blood toward the liver and spleen through the gastroduodenal artery (GDA). The GDA backfills the common hepatic artery such that retrograde flow in the common hepatic crosses the celiac artery origin to perfuse the splenic artery (Fig. 23.3). The finding of retrograde flow direction in the common hepatic artery is always associated with severe celiac artery stenosis or occlusion. Additionally, poststenotic turbulence in the splenic and hepatic arteries can be indirect signs of a celiac artery stenosis.
The SMA typically originates from the anterior surface of the aorta 1 to 2 cm below the celiac artery. It is best visualized in a sagittal plane as it courses parallel to the aorta with the left renal vein running between the SMA and aorta. The normal fasting SMA Doppler spectral waveform is triphasic (high resistance) with a brief phase of reversed flow followed by little or no flow in the second half of the cardiac cycle, similar to the pattern found in the major upper and lower extremity arteries (Fig. 23.4). The SMA should be scanned as far distally as possible, obtaining Doppler spectral waveforms from the proximal, middle, and distal segments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree