Medical Treatment of Heart Failure
The management of heart failure (HF) depends on the underlying mechanism. This chapter focuses on the treatment of HF related to left ventricular (LV) systolic dysfunction, the best understood and studied nonvalvular cause of HF. LV dysfunction is generally defined by a reduction in left ventricular ejection fraction (LVEF).
We briefly discuss lifestyle measures and then review established pharmacotherapy. Trial evidence is summarized where appropriate. Current guideline recommendations from the American College of Cardiology1 and the Heart Failure Society of America2 are cited in each section with some changes in wording for clarity. Where guidelines are quoted, the strength of the recommendation is given, followed by the level of evidence in parentheses (e.g., I,C refers to a class I recommendation with level of evidence C). Discussion of surgical treatments and device-based therapies for HF can be found elsewhere in this book.
LIFESTYLE MEASURES
Sodium Restriction
Retention of sodium is an important aspect of the pathophysiology of HF and occurs due to overactivation of the sympathetic and renin–angiotensin–aldosterone systems (see Chapter 15). It is felt for this reason that limitation of salt intake reduces HF symptoms. The typical recommendation is that patients adhere to a diet of <2 g/day of sodium from all sources.
ACC Guideline1
 Sodium restriction is indicated in patients with current or prior symptoms of HF and reduced LVEF who have evidence of fluid retention (I, C).
Sodium restriction is indicated in patients with current or prior symptoms of HF and reduced LVEF who have evidence of fluid retention (I, C).
Fluid Restriction
Fluid balance is generally monitored closely during hospitalization for HF. This can be difficult to achieve in the outpatient setting. For those with serum sodium levels <130, however, a fluid restriction of 2 L/day (and sometimes 1.5 L) may be advisable.
HFSA Guidelines2
 Restricted fluid intake is recommended for HF patients with moderate hyponatremia (serum Na <130) (I equivalent, C).
Restricted fluid intake is recommended for HF patients with moderate hyponatremia (serum Na <130) (I equivalent, C).
 Restricted fluid intake should be considered among HF patients without significant hyponatremia who demonstrate fluid retention that is difficult to control with sodium restriction and diuretic therapy (IIa equivalent, C).
Restricted fluid intake should be considered among HF patients without significant hyponatremia who demonstrate fluid retention that is difficult to control with sodium restriction and diuretic therapy (IIa equivalent, C).
Exercise
HF patients are generally counseled to engage in regular physical exercise within the limits of their symptoms. For those with a recent myocardial infarction (MI) or high burden of coronary artery disease, the usual recommendations on cardiac rehabilitation and exercise should be followed.
Clinical Trials
Multiple small trials had suggested that exercise training is associated with improved functional capacity, decreased HF hospitalizations, and possibly a decrease in mortality.3–5 In a meta-analysis, it has also been shown to have a favorable effect on LV remodeling.6
The HF-ACTION trial evaluated the effect of aerobic exercise in 2,331 patients with stable HF and LV dysfunction.7 Patients randomized to regular exercise had a greater improvement in peak oxygen consumption and 6-minute walk distance. In contrast to previous smaller trials, there was no difference in all-cause mortality or hospitalization after a mean follow-up of 30 months.
ACC Guideline1
 Exercise training is recommended as an adjunctive approach to improve clinical status in ambulatory patients with current or prior symptoms of HF and reduced LVEF (I, B).
Exercise training is recommended as an adjunctive approach to improve clinical status in ambulatory patients with current or prior symptoms of HF and reduced LVEF (I, B).
MEDICAL THERAPY IN CHRONIC HEART FAILURE
Neurohormonal Blockade
Activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system leads to adverse physiologic effects in HF. Medications that block or dampen these effects include beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, AT1 receptor blockers (ARBs), and aldosterone antagonists. These medications have become an important cornerstone of contemporary HF management. It is recommended that these agents, particularly ACE inhibitors and beta-blockers, be considered in all HF patients and that they be titrated to achieve the doses used in clinical trials or to maximal tolerated doses.
Beta-Blockers
Mechanism of Action There are a number of proposed mechanisms by which beta-adrenergic blockers have been theorized to be beneficial in HE. Over time, they improve the efficiency of beta-adrenergic signaling, and some agents in this class restore a more normal distribution of beta-receptors on the cell surface. Hemodynamic and cellular effects include reduced wall tension, inhibition of adverse remodeling, and prevention of myocyte apoptosis.8 Beta-blockers have a favorable impact on ventricular remodeling in both ischemic and nonischemic cardiomyopathies.9
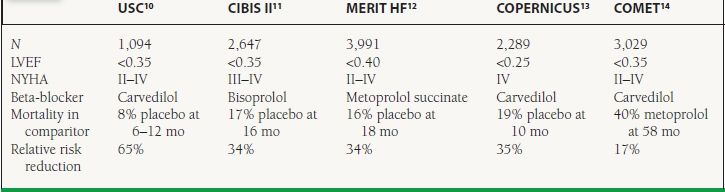
Clinical Trials Multiple large-scale RCTs have shown beta-blockers to improve symptoms and reduce mortality in HF (Table 16.1).10–14 It should be noted that the nonselective beta-blocker bucindolol was also studied in the BEST trial, which showed no significant improvement in mortality.15 This agent has a significant degree of intrinsic sympathomimetic activity.
TABLE
16.1 Landmark Trials Evaluating the Effect of Beta-Blockers on Clinical Outcomes in HF
Cautions
 Bradycardia can occur due to sinus node slowing and/or AV block.
Bradycardia can occur due to sinus node slowing and/or AV block.
 Initiation of beta-blockers during acute HF decompensation can worsen symptoms due to negative inotropy.
Initiation of beta-blockers during acute HF decompensation can worsen symptoms due to negative inotropy.
ACC Guidelines1
 Beta-blockers are indicated in patients with current or prior symptoms of HF and reduced LVEF (I, A).
Beta-blockers are indicated in patients with current or prior symptoms of HF and reduced LVEF (I, A).
 Beta-blockers are indicated in patients with asymptomatic LV dysfunction (I, A).
Beta-blockers are indicated in patients with asymptomatic LV dysfunction (I, A).
 Agents demonstrated to be beneficial in HF include carvedilol, bisoprolol, and metoprolol succinate.
Agents demonstrated to be beneficial in HF include carvedilol, bisoprolol, and metoprolol succinate.
Angiotensin Converting Enzyme Inhibitors
Mechanism of Action These agents block the ACE, preventing conversion of angiotensin I to angiotensin II (see Fig. 6.3). The role of angiotensin II in HF is discussed in detail in Chapter 15. By preventing the formation of angiotensin II, ACE inhibitors decrease vasoconstriction, increasing arterial compliance. The resulting decrease in vascular resistance unloads the failing left ventricle. ACE inhibitors reduce adverse structural changes such as ventricular hypertrophy and dilation, even among patients without overt HF.16,17 At a microscopic level, they have also been shown to reduce fibrosis.18
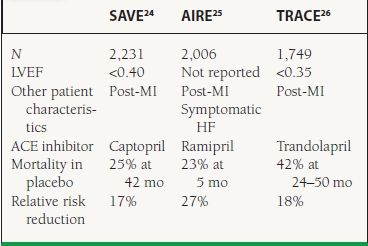
Clinical Trials Numerous published trials have demonstrated reductions in cardiovascular endpoints including mortality using ACE inhibitors in patients across the spectrum of HF (Table 16.2).19–23 ACE inhibitors have also been studied extensively in the setting of MI complicated by LV dysfunction or HF. Three key trials are summarized in Table 16.3.24–26
TABLE
16.2 Landmark Trials of ACE Inhibitors in chronic HF or LV Dysfunction
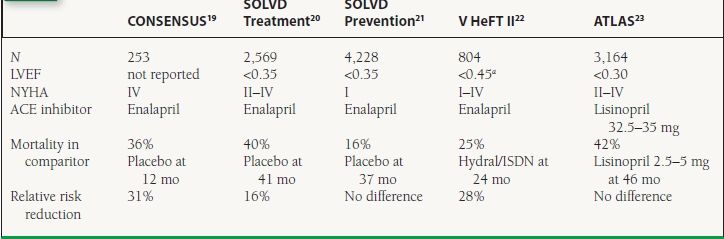
aSome patients were enrolled on the basis of increased LV end-diastolic diameter or cardiothoracic ratio; mean LVEF was 0.29. ISDN = isosorbide dinitrate.
TABLE
16.3 Landmark Trials of ACE Inhibitors in LV Dysfunction or HF Post-MI
Cautions
 Angioedema is a rare but serious complication of ACE inhibitors.
Angioedema is a rare but serious complication of ACE inhibitors.
 Hyperkalemia can occur, especially in combination with ARBs or spironolactone.
Hyperkalemia can occur, especially in combination with ARBs or spironolactone.
 Renal dysfunction can be exacerbated by ACE inhibitors.
Renal dysfunction can be exacerbated by ACE inhibitors.
 Known bilateral renal artery stenosis is a contraindication to ACE inhibitors.
Known bilateral renal artery stenosis is a contraindication to ACE inhibitors.
 ACE inhibitors should not be used during pregnancy.
ACE inhibitors should not be used during pregnancy.
 ACE inhibition leads to increased levels of bradykinin—in some patients, this is associated with the development of dry cough.
ACE inhibition leads to increased levels of bradykinin—in some patients, this is associated with the development of dry cough.
ACC Guidelines1
 ACE inhibitors are indicated in patients with current or prior symptoms of HF and reduced LVEF (I, A).
ACE inhibitors are indicated in patients with current or prior symptoms of HF and reduced LVEF (I, A).
 ACE inhibitors should be used in patients with reduced LVEF in the absence of symptoms (I, A).
ACE inhibitors should be used in patients with reduced LVEF in the absence of symptoms (I, A).
Angiotensin Receptor Blockers
Mechanism of Action Angiotensin can be produced by other proteases besides ACE. Furthermore, although angiotensin II action on AT1 receptors leads to vasoconstriction and adverse remodeling, action on AT2 receptors may actually have beneficial antifibrotic effects.27 These findings helped suggest the idea that using a selective ARB may be beneficial in HF.
Clinical Trials—ACE Inhibitor-Intolerant Patients The CHARM Alternative trial enrolled 2,028 patients with HF who were intolerant of ACE inhibitors and randomized them to candesartan or placebo.28 There was a significant reduction in the rate of the primary endpoint of death or HF hospitalization at 34 months in the candesartan group (33% vs. 40% with placebo).
Clinical Trials—Comparison to ACE Inhibitors The data from head to head trials comparing ACE inhibitors and ARBs in HF are mixed (Table 16.4). The ELITE I trial suggested a significant hard-endpoint benefit of using losartan rather than captopril in HF.29 This finding was not reproduced in the larger follow-up ELITE II trial.30 The OPTIMAAL study in post-MI patients showed the opposite result with a trend toward captopril being superior to losartan with respect to major cardiovascular events.31 The VALIANT study compared the ACE inhibitor captopril, the ARB valsartan, and a combination of the two in post-MI patients with HF or LV dysfunction.32 In this study, there was no difference between groups with respect to rates of death or HF hospitalization.
TABLE
16.4 Landmark Trials Comparing ARBS to ACE Inhibitors in HF
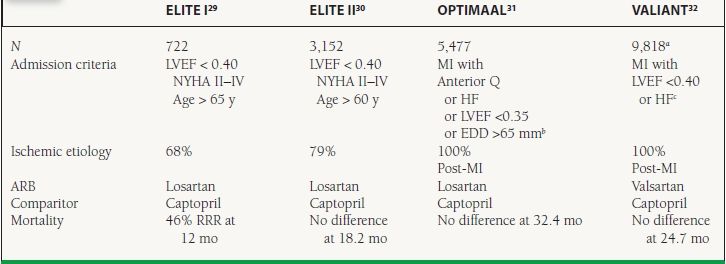
a4,909 valsartan and 4,909 captopril; combination arm with 4,885 excluded here.
EDD=end diastolic diameter; LV LVEF <0.35 or EDD >65 mm present in only 13.6% of patients.
c28% had no symptoms and mean LVEF was 0.35.
Clinical Trials—Combination with ACE Inhibitors Several trials have tested the effects of adding ARB therapy to patients already stabilized on ACE inhibitors (Table 16.5). CHARM added randomized patients already taking ACE inhibitors to the addition of candesartan or placebo.33 This trial showed a significant reduction in death and HF hospitalization in patients receiving candesartan. The ValHeft trial randomized patients to valsartan or placebo in addition to regular HF therapy.34 More than 92% of patients were on an ACE inhibitor at baseline which was continued. In the overall trial, there was no mortality difference between groups, but patients receiving valsartan had a significant reduction in HF hospitalization. As mentioned above, the VALIANT study compared the combination of valsartan and captopril to the use of either agent alone.32 The 4,885 patients who received the combination of ACE inhibitor and ARB had mortality rates and HF hospitalization rates that were identical to those of the captopril alone group.
TABLE
16.5 Landmark Trials of the Addition of an ARB to an ACE Inhibitor in HF
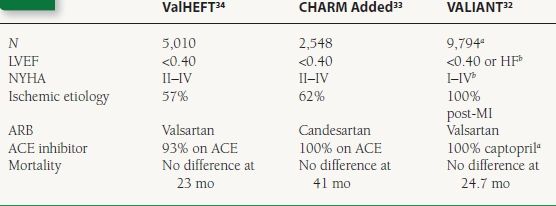
a4,909 captopril alone and 4,885 combination; 4,909 patients on valsartan alone excluded here.
bRequired either LVEF < 0.40 or overt HF; 28% had no symptoms and mean LVEF was 0.35.
Cautions
 Angioedema can occur with ARBs but much less commonly than with ACE inhibitors.
Angioedema can occur with ARBs but much less commonly than with ACE inhibitors.
 Cough caused by ACE inhibitors is not seen with ARBs.
Cough caused by ACE inhibitors is not seen with ARBs.
Otherwise, the side effect profile of ARBs is much like that of ACE inhibitors.
ACC Guidelines1
 ARBs are indicated for ACE inhibitor-intolerant patients with HF and reduced LVEF (I, A).
ARBs are indicated for ACE inhibitor-intolerant patients with HF and reduced LVEF (I, A).
 In patients with reduced LVEF but no symptoms of HF who are intolerant of ACE inhibitors, an ARB should be used if the etiology is ischemic (I, B) and is reasonable if the etiology is nonischemic (Ila, C).
In patients with reduced LVEF but no symptoms of HF who are intolerant of ACE inhibitors, an ARB should be used if the etiology is ischemic (I, B) and is reasonable if the etiology is nonischemic (Ila, C).
 ARBs are a reasonable first-line alternative to ACE inhibitors in patients with HF and reduced LVEF (Ila, A).
ARBs are a reasonable first-line alternative to ACE inhibitors in patients with HF and reduced LVEF (Ila, A).
 The addition of an ARB to an ACE inhibitor and beta-blocker can be considered in persistently symptomatic HF patients with reduced LVEF (IIb, B).
The addition of an ARB to an ACE inhibitor and beta-blocker can be considered in persistently symptomatic HF patients with reduced LVEF (IIb, B).
Aldosterone Antagonists
Mechanism of Action Spironolactone was originally used as a potassium-sparing diuretic. It blocks mineralocorticoid receptors in the kidney, preventing reabsorption of sodium and excretion of potassium in the distal convoluted tubule. Because aldosterone production is upregulated in HF (see Chapter 15), it becomes an obvious target for therapies. Spironolactone also has some androgen blocking activity, which can lead to adverse effects such as breast tenderness and gynecomastia. Eplerenone is more selective for the aldosterone receptor and does not have these same side effects.
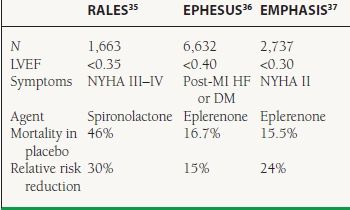
Clinical Trials The RALES trial firmly established the benefits of aldosterone antagonism in patients with severe symptomatic HF and reduced LVEF35 The EPHESUS trial studied only post-MI patients with resulting LV dysfunction.36 In an effort to include higher risk patients, the investigators required patients to have either symptomatic HF or diabetes. The recently published EMPHASIS study may expand the use of these agents in patients with less symptomatic HF, although these findings have yet to be incorporated into national guidelines.37 These three trials are summarized in Table 16.6.
TABLE
16.6 Landmark Trials of Aldosterone ALandmark Trials of Aldosterone and Post-MI LV Dysfunction
Cautions
 Caution should be used in patients with renal dysfunction.
Caution should be used in patients with renal dysfunction.
 Hyperkalemia (Should not be prescribed if K > 5 mM)
Hyperkalemia (Should not be prescribed if K > 5 mM)
 Spironolactone can be associated with breast tenderness and gynecomastia.
Spironolactone can be associated with breast tenderness and gynecomastia.
ACC Guidelines1
 An aldosterone antagonist is recommended in selected patients with NYHA III or IV symptoms of HF and reduced LVEF who can be monitored for preserved renal function and normal potassium concentration (I, A).
An aldosterone antagonist is recommended in selected patients with NYHA III or IV symptoms of HF and reduced LVEF who can be monitored for preserved renal function and normal potassium concentration (I, A).
 Creatinine should be ≤2.5 in men or ≤2.0 mg/dL in women.
Creatinine should be ≤2.5 in men or ≤2.0 mg/dL in women.
 Potassium should be ≤5.0 mEq/L.
Potassium should be ≤5.0 mEq/L.
Other Vasodilators
Afterload reduction of the LV in chronic HF decreases wall tension and improves forward stroke volume. Preload reduction with venous vasodilator therapy helps to decrease LV filling pressures and improve symptoms.
Hydralazine and Nitrates
Mechanism of Action Hydralazine increases intracellular cGMP promoting smooth muscle relaxation leading to vasodilation. It acts primarily in the arterioles, decreasing blood pressure and LV afterload. It is often given in conjunction with nitrates in HF because of a synergistic activity on the nitric oxide pathway and more sustained clinical effect. The combination of hydralazine and a nitrate leads to lower LV filling pressure and an increase in cardiac output.
Clinical Trials The original V-HeFT study compared a combination of hydralazine and isosorbide dinitrate (ISDN) with placebo in patients with HF and reduced LVEF.38 It showed a significant mortality reduction to 26% at 2 years from 34% in the placebo arm.
V-HeFT II compared the same combination of hydralazine and ISDN to the ACE inhibitor enalapril.22 Event rates in the hydralazine and ISDN arm of V-HeFT II were similar to those of V-HeFT, with a 2-year mortality of 25%. The enalapril arm, however, had an even lower mortality rate of 18% at 2 years.
In the V-HeFT II trial, there appeared to be less benefit of ACE inhibitor over hydralazine and nitrates among black patients. The A-HeFT trial enrolled patients self-described as African American to directly test the utility of adding hydralazine and ISDN when symptoms persisted on ACE inhibitors.39 The trial was discontinued early after a mean follow-up of 10 months because of a significant reduction in mortality in the group treated with hydralazine and ISDN (6.0% vs. 10.2% in placebo).
Cautions
 Use of phosphodiesterase inhibitors such as sildenafil for erectile dysfunction in combination with nitrates is contraindicated because of the risk of hypotension.
Use of phosphodiesterase inhibitors such as sildenafil for erectile dysfunction in combination with nitrates is contraindicated because of the risk of hypotension.
 Methemoglobinemia is a rare but serious side effect of nitroglycerin.
Methemoglobinemia is a rare but serious side effect of nitroglycerin.
 Headache is a common side effect of nitrates.
Headache is a common side effect of nitrates.
ACC Guidelines1
 Hydralazine and a nitrate could be considered in patients with HF and decreased LVEF who are intolerant of ACE inhibitors and ARBs (IIb, C).
Hydralazine and a nitrate could be considered in patients with HF and decreased LVEF who are intolerant of ACE inhibitors and ARBs (IIb, C).
 Hydralazine and a nitrate should be used in self-identified African American patients with HF and decreased LVEF who have persistent moderate or severe symptoms despite treatment with a beta-blocker, an ACE inhibitor, and a diuretic (I, B).
Hydralazine and a nitrate should be used in self-identified African American patients with HF and decreased LVEF who have persistent moderate or severe symptoms despite treatment with a beta-blocker, an ACE inhibitor, and a diuretic (I, B).
Calcium Channel Blockers
Mechanism of Action In general, calcium channel blockers are not recommended in HF and impaired LV function. The more cardioselective diltiazem and verapamil in particular are contraindicated because of their negative inotropic properties. The dihydropyridines, on the other hand, act more peripherally causing vasodilation and reducing blood pressure.
Clinical Trials The MDPIT trial evaluated the benefits of diltiazem in patients who had suffered a MI. Among patients with pulmonary edema on chest x-ray, use of diltiazem was associated with higher cardiac mortality.40 The PRAISE-I study compared amlodipine to placebo in patients with HF and reduced LVEF41 It showed a trend toward a decrease in the combined endpoint of death or CV hospitalization among patients randomized to amlodipine. This difference was significant among the subset of patients with a nonischemic cardiomyopathy. The subsequent PRAISE-II trial enrolled only patients with nonischemic-dilated cardiomyopathies and randomized them to amlodipine or placebo. There was no difference in outcomes found. Small randomized trials of amlodipine42 and nifedipine43 have shown mixed results with respect to exercise tolerance with these agents.
Cautions
 Nondihydropyridine calcium channel blockers diltiazem and verapamil have negative inotropic effects and are considered contraindicated in HF.
Nondihydropyridine calcium channel blockers diltiazem and verapamil have negative inotropic effects and are considered contraindicated in HF.
 Amlodipine and nifedipine can be associated with edema and increased fluid retention.
Amlodipine and nifedipine can be associated with edema and increased fluid retention.
ACC Guidelines1
 Calcium channel blockers should not be used as routine treatment in patients with current or prior symptoms of HF and reduced LVEF (III, A).
Calcium channel blockers should not be used as routine treatment in patients with current or prior symptoms of HF and reduced LVEF (III, A).
Alpha-blockers
The ALLHAT trial showed increased rates of HF in patients randomized to the alpha-blocker doxazosin compared to other antihypertensives.44 Alpha-blockers continue to be used as add-on therapy in patients with HF and refractory hypertension despite maximal doses of indicated drugs. The use of alpha-blockers in HF is not specifically mentioned in the ACC or HFSA guidelines.
Digitalis Glycosides
Preparations of digitalis have been used for centuries in the treatment of cardiovascular diseases. Digoxin is primarily used for rate control of atrial fibrillation, but it has an important role in the management of symptomatic HF in selected patients in sinus rhythm as well.
Digoxin
Mechanism of Action Digoxin acts via a number of different mechanisms. The primary effect has long been felt to be blockade of the Na/K ATPase in cardiac myocytes (see Chapter 6). This leads to an increase in intracellular calcium available for contraction and therefore to an increase in inotropy. Effects in other tissues lead to digoxin having sympatholytic and parasympathomimetic effects. It is now felt that the neurohormonal blockade activities of digoxin may play an important role in the long-term clinical benefits of its use.
Clinical Trials The Digitalis Investigation Group (DIG) trial studied the effects of digoxin in comparison to placebo in 6,800 patients with HF and decreased LVEF45 There was no significant difference between groups in the rates of mortality at a mean follow-up of 37 months. There was, however, a significant decrease in hospitalization for worsening HF in the digoxin group (26.8% vs. 34.7% in placebo).
Withdrawal Trials Several trials have tested the effects of withdrawing digoxin from stable patients on more contemporary HF treatment. The PROVED study randomized 88 patients with symptomatic HF on digoxin to continuation or withdrawal.46 Discontinuation of digoxin resulted in higher rates of treatment failure and decreased exercise tolerance. The RADIANCE study similarly randomized 178 patients to continuation of digoxin or withdrawal of the agent for 12 weeks and initiation of placebo.47 Patients in the placebo arm had higher rates of crossover to active treatment. In this study, they also showed significant worsening of symptoms and exercise tolerance.
Monitoring of Levels Digoxin toxicity is a major concern among HF patients, particularly the elderly and those prone to electrolyte disturbances. Digoxin toxicity is most common with serum levels >2.0, but can occur at lower levels. A post hoc analysis of the DIG trial demonstrated an interaction between serum digoxin levels and all-cause mortality.48
Cautions
 Digoxin toxicity is a life-threatening complication of treatment with numerous manifestations.
Digoxin toxicity is a life-threatening complication of treatment with numerous manifestations.
 Hypokalemia, hypomagnesemia, hypercalcemia, and acute renal failure can all precipitate digoxin toxicity.
Hypokalemia, hypomagnesemia, hypercalcemia, and acute renal failure can all precipitate digoxin toxicity.
ACC Guideline1
 The use of digoxin is reasonable in patients with current or prior symptoms of HF and decreased LVEF (IIa, B).
The use of digoxin is reasonable in patients with current or prior symptoms of HF and decreased LVEF (IIa, B).
HFSA Guideline2
 Serum digoxin levels should be maintained <1.0 ng/mL (generally 0.7 to 0.9 ng/mL).
Serum digoxin levels should be maintained <1.0 ng/mL (generally 0.7 to 0.9 ng/mL).
Investigational Agents
Many other agents have been tested or are under investigation for the treatment of HF. Several agents and classes with recent publications are briefly discussed below.
Phosphodiesterase Inhibitors
LV dysfunction with HF is a major secondary cause of pulmonary hypertension (type 2 by the current classification scheme). There are theoretical concerns that reducing pulmonary pressures in patients with left-sided HF could lead to increased pulmonary edema. Nevertheless, there is interest in using selective pulmonary arterial vasodilators in HF. Several small trials have shown hemodynamic benefit and increased exercise tolerance.49,50
Polyunsaturated Fatty Acids
Epidemiologic studies have linked consumption of polyunsaturated fatty acids (PUFAs) with decreased rates of adverse cardiovascular events.51 GISSI-HF randomized 6,975 patients with symptomatic HF (>90% of whom had LVEF < 0.40) to omega-3 PUFA or placebo.52 At a follow-up of 3.9 years, there was a statistically significant reduction in allcause mortality (27% vs. 29% in placebo).
Ivabradine
This medication has selective inhibitory effects on the sinus node. It was postulated as the basis of the SHIFT study that controlling heart rate with such an agent in HF patients unable to tolerate maximal beta-blocker doses may be beneficial. SHIFT randomized 6,558 patients to receive ivabradine or placebo in addition to conventional HF therapy.53 Although there was a significant reduction in cardiovascular death or HF hospitalization, the trial has been criticized for underdosing of beta-blockers. The argument is that slowing the sinus node rate may be beneficial but could have been achieved by uptitration of beta-blockers to maximal doses.
Treatment of Related Conditions
Anemia and Iron Deficiency
Anemia is commonly associated with HF, and normalization of hemoglobin may be associated with increased exercise tolerance. There is evolving evidence to suggest that there may be symptomatic benefit to treatment of iron deficiency in HF even in the absence of anemia.
Clinical Trials The STAMINA-HF trial examined the effects of darbapoetin alpha in 319 patients with HF and anemia.54
Treatment resulted in a significant increase in hemoglobin levels but had no significant impact on mortality or hospitalization rates.
FAIR-HF enrolled 459 patients with symptomatic HF and decreased LVEF who had iron deficiency defined as ferritin <100 µg/L (or <300 µg/L with transferrin saturation <20%).55 Only half of the patients had anemia (defined as hemoglobin <12.0 g/dL). Randomization was to a regimen of intravenous iron to achieve iron repletion or placebo. Iron therapy was associated with improved self-reported quality of life and symptom status at 24 weeks, both among anemic and nonanemic irondeficient HF patients.
ACC Guidelines1
 Increasing erythropoiesis in HF patients with decreased LVEF is not well established (IIb, B).
Increasing erythropoiesis in HF patients with decreased LVEF is not well established (IIb, B).
 Iron supplementation therapy “is undergoing further investigation.”
Iron supplementation therapy “is undergoing further investigation.”



