Fig. 21.1
The three types of SBS after surgical resection: type I (end-jejunostomy), type II (jejunocolonic anastomosis), and type III (ileocolonic anastomosis)
Intestinal adaptation depends on multiple factors, including the extent and site of intestinal loss or dysfunction, the function of the remaining small bowel and associated digestive organs, the presence of the ICV and thus terminal ileum and colon, and the amount of time that has elapsed since resection. The adaptive process begins within 12–24 h after resection and continues for 1–2 years [5, 12, 13]. The process of adaptation involves the lengthening, dilation, growth of villi, deepening of the crypts, and enterocyte hyperplasia [14]. The combined effect of all of these mechanisms increases the surface area and enhances the absorption of nutrients and electrolytes (Fig. 21.2). Loss of the duodenum or the terminal ileum, in particular the ileocecal valve, impairs absorption much more than loss of other parts of the small bowel. Both the duodenum and the ileocecal region possess specific absorptive functions and play a crucial role in the regulation and integration of postprandial gastrointestinal motility and secretion. The ileum better compensates for jejunal loss; the jejunum in contrast is less adaptable to the loss of the ileum [9–11, 15]. However, any adaptive mechanism can be overwhelming, and adaptation can be inadequate if too much small bowel is lost. Although length alone is not the only determining factor of complications related to small bowel resection, resection of up to 70 % of the small bowel is usually well tolerated if the terminal ileum and ileocecal valve are preserved.
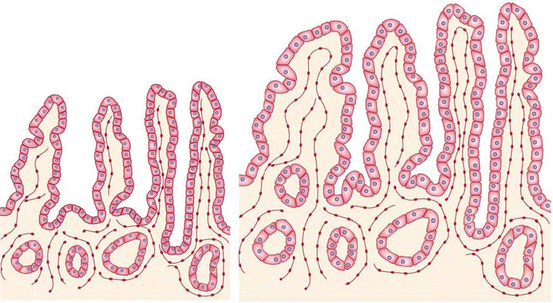

Fig. 21.2
This figure shows the lengthening, dilation, growth of villi, deepening of the crypts, and enterocyte hyperplasia during intestinal adaptation. The left side of the figure shows an intestinal epithelium before adaptation; the right side shows an intestinal epithelium after adaptation
Phases of Short Bowel Syndrome
The clinical phase of SBS can be divided into three phases: early phase, intermediate phase, and late phase. The early phase (first few days after the resection) is mainly characterized by watery diarrhea resulting in dehydration, hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia. An intermediate phase occurs from 1 week to approximately 1 year during which intestinal adaptation occurs. During the late phase, after maximal intestinal adaptation has been achieved, weight often stabilizes. After surgery, fluid losses may exceed 5 L/day, especially with concomitant colectomy [16, 17]. Gastric hypersecretion evokes intestinal mucosal damage, impaired micelle formation, and inhibition of pancreatic enzyme function.
Treatment of Short Bowel Syndrome
The management of SBS is different in the three phases. The management in the early phase involves intensive postoperative care and revolves around control and treatment of sepsis, maintenance of fluid and electrolytes, and nutritional support. The management in the intermediate phase is to initiate nutritional support (enteral, parenteral, and oral) with the goal of maximizing intestinal adaptation. During the late phase, no further improvement in nutrition can be achieved, and this time the goal is to provide adequate nutrition and prevent complications. The summary of management of SBS in all three phases is given in Table 21.1.
Table 21.1
Management strategies
Early phase |
Treat postoperative complications |
Diet modification to limit the output, diarrhea |
ORS |
Document the remaining bowel length |
Assess the need for TPN/HPN |
Intermediate phase |
Pharmacotherapy (choice depends on the type of SBS) |
Diet modification, ORS |
Train for long-term TPN, set goals for weaning HPN |
Assess bowel adaptation |
Late phase |
Monitor for QOL on HPN |
Prevent complications on HPN |
Other treatment options |
Nutritional Strategies
Optimizing and maintaining nutritional status is important for adaptation subsequent to surgery, and it is central to long-term survival. Most patients in the early phase require total parenteral nutrition (TPN). TPN and home parenteral nutrition (HPN) might be required in the intermediate and late phases too. Prior to the introduction of PN in the early 1970s, most patients with SBS died of severe malnutrition, dehydration, and weight loss. The 1- and 5-year survival of patients on TPN ranges from 91 % to 97 % in adults. Nutritional strategies include dietary modification, optimizing TPN and initiating enteral nutrition, and oral feeding when possible.
Dietary Modifications
The proximal small bowel receives approximately nine liters of water and electrolytes daily from various sources, of which 90 % are reabsorbed. This reabsorption is significantly impaired in SBS patients. Thus, a substantial dietary modification is necessary in these patients to prevent diarrhea and dehydration [18]. It is a general opinion that polyphagia is required to meet the excess output from the gastrointestinal tract, but the authors propose that it is more important to choose the right type of food with low osmolarity to decrease the output. For most patients suffering from SBS, dietary strategies include small portions of frequent meals consisting of complex carbohydrates (40–60 % of the total energy requirement) and protein (20–30 % of the total energy requirement) while limiting simple sugars. In SBS patients, drinking regular water, juice, or sports drinks will also worsen the diarrhea. Liquids that have a high concentration of sugar and salt will cause osmotic fluid shift to the intestinal lumen. The additional fluid in the lumen will exacerbate the diarrhea and worsen the dehydration [6]. A list of food items and drinks that are recommended to be avoided by SBS patients is presented in Table 21.2. Proper guidance and recommendations should be provided to all patients with SBS to decrease diarrhea and improve symptoms. When a particular drink cannot be completely avoided, often diluting it with water can be recommended. For example, dilution of one cup of cranberry juice or regular soda with two cups of water can decrease the concentration of the drink considerably and provide a favorable osmolarity profile. Water, tea, coffee, and diet soda pop are considered as “free water” and should also be avoided by SBS patients. Because these fluids have small amounts of sodium, the sodium from the bloodstream moves into the intestinal lumen causing water to follow worsening diarrhea [6]. The diet requirements and recommendations may also vary with different types of SBS. SBS type II and type III patients with intact colon can be fed higher proportions of complex carbohydrates and medium-chain triglycerides. Oxalate restriction is also advised in these patients [19, 20]. SBS type I patients with the colon removed do not require an oxalate restriction and can be fed long-chain triglycerides and complex carbohydrates.
Table 21.2
List of common fluids that might exacerbate the fluid output in SBS patients
Fruit juices |
Regular soda pop |
Nutritional supplements such as Boost™, Boost Plus™, Boost Breeze™, Carnation Instant Breakfast™ (including sugar-free Carnation Instant Breakfast™) in powder form, Enlive™, Ensure™, Ensure Plus™, Glucerna Shake™, Resource™, Resource™ Fruit Beverage, Sportshake™ |
Fruit drinks such as Hi-C™, SunnyD™, Tang™ |
Sports drinks such as Gatorade™ or Powerade™ |
Oral Rehydration Solution
Oral rehydration solution (ORS) is a scientifically formulated blend of carbohydrates, salts, and water developed to treat dehydration. ORS works by effectively controlling the primary water absorption through osmosis. The effectiveness of ORS improved when the polymers of glucose in the ORS were replaced by various forms of simple glucose. Rice powder/rice syrup has been shown to effectively replace the standard glucose in ORS [6]. This change decreased the osmolarity of the ORS and increased the ratio of glucose to sodium. In the authors’ clinical practice, ORS plays an integral part in the treatment of high stomal outputs of patients with SBS. Over the years, it has been realized that with adequate dietary strategies and improved ORS use, it is possible to avoid turning to parenteral solutions to maintain fluid. The details of the WHO recommended formula for preparing ORS is presented in Table 21.3. Electrolyte details and osmolarity of some of the commercially available ORS preparation are presented in Table 21.4. Despite these oral restrictions and recommendations, patients with severe SBS often still require parenteral rehydration with daily runs of intravenous saline.
Table 21.3
Various electrolyte contents, sugar contents, and osmolarity of commercial ORS solution
Solution | Type of sugar | Carbo-hydrates (g/L) | Sodium (mEq/L) | Potassium (mEq/L) | Magnesium (mEq/L) | Osmolarity (mOsm) |
|---|---|---|---|---|---|---|
WHO ORS | Glucose | 20 | 90 | 20 | 0 | 330 |
Pedialyte® | Dextrose, acesulfame | 25 | 45 | 20 | 0 | 250 |
CeraLyte 75® | Rice syrup solids, rice syrup | 40 | 75 | 20 | 0 | <250 |
CeraLyte 90® | Rice syrup solids, rice syrup | 40 | 90 | 20 | 0 | <275 |
DripDrop® | Sucrose, fructose, sucralose | 33 | 60 | 20 | 13.7 | 235 |
Table 21.4
Modified WHO ORS recipe
Baking soda (sodium bicarbonate) – ½ teaspoon |
Salt substitute (potassium chloride) – ¼ teaspoon |
Table salt (sodium chloride) – ½ teaspoon |
Sugar (sucrose) – 2 tablespoons |
Tap water – add enough to make 1 l |
Tip: patients can be recommended to flavor it with sugar-free drink mixes and artificial sweeteners to improve the taste |
Home Parenteral Nutrition
Total parenteral nutrition (TPN) is vital in the early phase of SBS treatment. TPN is also required in many SBS patients during the intermediate and late phases. If required during the intermediate and late SBS phases, patients can go home on PN [21]. It is essential that the home parenteral nutrition (HPN) is administered by a multidisciplinary team of nutrition experts. HPN aims both to prevent and restore nutritional deficits while minimizing the complications related to the therapy itself. When sending a patient on HPN, it is essential to ensure that the benefits will outweigh the associated risks [21]. In addition, it is important to identify small intestine adaptation (e.g., less fluid and calorie requirements) and make necessary changes in the HPN infusate. One of the most important decisions with initiation of HPN is to determine duration of the intended therapy and taking necessary steps to reach this goal. This will guide the HPN team and patients in realistic expectations for a time frame for weaning off HPN. Furthermore, this can serve as a guide for providing proper training techniques and choice of central venous access devices. For short-term (6 weeks) TPN and eventually HPN, a peripherally inserted central catheter (PICC) can be used. Usually, when the HPN is required for more than 6 weeks, a single lumen Hickman® or implanted port is more ideal. PICC lines carry a greater risk of thrombosis and catheter-related blood stream infection probably due to multiple lumens, smaller diameter, and greater overall length. Some advantages of a port are minimal alteration in body image, no concern for accidental pulling or cutting of the device, and ability to swim in lakes. However, when removal is needed, ports have to be surgically removed. It is for this reason that many of the specialized centers managing HPN use single lumen Hickman® catheter.
Complicated cases of SBS that were previously thought to be terminal because of the extremely short bowel are now manageable with HPN. With the introduction of small portable pumps equipped with infusion rate monitors, it is now possible to administer HPN at night over shorter periods of time (12 h instead of 24 h), giving patients the freedom from being connected for 24 h. This has led to more independence with the ability to maintain employment, travel, and live at home instead of skilled nursing facilities. There is some difficulty in capturing and defining QOL in HPN patients. This is because it is difficult to determine the impact on QOL from symptoms related to the primary disease vs HPN. Although there are numerous complications of HPN (e.g., infection, thrombosis, liver disease, and metabolic bone disease), for many SBS patients it is lifesaving.
Pharmacologic Treatments
One of the main complaints in patients with SBS is diarrhea due to rapid transit. Pharmacological agents can be used in SBS patients to slow down the small intestine’s transit time. These agents can be divided into subgroups based on their mechanisms of action including antidiarrheals, antisecretory, and growth factors. A combination of these three general classes may be needed to achieve optimal results in SBS patients. A list of common drugs used in SBS patients, their recommended dosage, and common side effects is presented in Table 21.5.
Table 21.5
Various drugs, recommended maximum dosage, and adverse effects
Drug | Maximum recommended dosage | Adverse effects |
|---|---|---|
Loperamide | 32 mg/day | Nausea, toxic megacolon, angioedema |
Diphenoxylate-atropine | 30 mg/day | Confusion, dry mouth, lethargy, dizziness, drug dependence
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|