OBJECTIVES AND INDICATIONS FOR MECHANICAL VENTILATION
GOALS OF MECHANICAL VENTILATION
Mechanical ventilation is the process by which a device supports the partial or total transport of oxygen and carbon dioxide between the environment and the pulmonary capillary bed. Mechanical ventilation itself is not a treatment modality, but rather a measure to restore effective gas exchange and reduce the patient’s work of breathing as the underlying pathology is being treated. The goals of mechanical ventilation are listed in
Table 27.1.
INDICATIONS FOR MECHANICAL VENTILATION
The indications for ventilatory support are best understood after one characterizes the causes of respiratory failure into either hypoxemic or hypercapnic categories (
Table 27.2).
Most of the patients being admitted to a cardiac care unit (CCU) will fall into the category of hypoxemic respiratory failure caused by cardiogenic pulmonary edema, which leads to decreased lung compliance, ventilation-perfusion mismatching, and diffusion impairment. Hypoxemia of mild to moderate severity can often be managed by the administration of oxygen through delivery systems ranging from nasal cannula to face masks. However, it can be increasingly difficult to maintain adequate oxygenation and oxygen delivery with more severe hypoxemia, and these patients may require positive-pressure ventilation as they are commonly in considerable distress.
Physiologic derangements and clinical findings will determine the need for mechanical ventilation, which should be considered early in the course and not be delayed until the need becomes emergent. Patients suffering from cardiogenic shock will have an increased respiratory workload and reduced cardiac output delivered to the muscles of respiration. They are often described as “tiring out” or developing respiratory muscle fatigue and may show physical exam findings such as nasal flaring, tracheal tug, recruitment of accessory muscles of respiration (sternocleidomastoids or intercostals), paradoxical or asynchronous movements of the rib cage and abdomen, and an increased pulsus paradoxus. If the mechanical workload progressively increases, the breathing demand at some point will exceed the capabilities of respiratory pump. The patient will be unable to sustain adequate levels of ventilation to effectively eliminate carbon dioxide, and progressive hypercapnia will ensue.
A substantial proportion of patients who require mechanical ventilation have relatively normal arterial blood gases but show the other signs of respiratory failure mentioned earlier. Therefore,
the decision to place a patient on mechanical ventilation should not be determined by abnormalities of the arterial blood gas.
Moreover, increased respiratory work may increase the oxygen cost of breathing to as much as 50% of total body oxygen consumption. When this occurs, the respiratory muscles disproportionally consume oxygen at the expense of aerobic metabolism of other vital organs such as the heart, brain, and kidneys. Under these circumstances, mechanical ventilation decreases the work of breathing, decreases the oxygen cost of breathing, and redistributes oxygen delivery to the respiratory system thereby improving oxygen delivery to other bodily organs.
1,
2If the underlying pathology is only transient or readily reversible, ventilation may be achieved through noninvasive modes. However, those patients who are postcardiac arrest, undergoing therapeutic hypothermia, experiencing ongoing cardiac ischemia, demonstrating cardiac or airway instability, exhibiting altered mental status, or having copious secretions, are poor candidates for noninvasive ventilation (NIV) and they require endotracheal intubation with positive-pressure ventilation.
NONINVASIVE VENTILATION
NIV refers to a method of delivering ventilatory assistance with the use of a mask interface rather than with an invasive interface, such as an endotracheal tube (ETT) or tracheostomy. NIV may provide respiratory support to selected patients while avoiding complications of invasive mechanical ventilation.
3,
4 The modes that are most commonly used in the critical care setting are continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BPAP).
COMPONENTS OF THE NONINVASIVE VENTILATOR
The application of NIV requires a nasal or oronasal mask interface and a device that is capable of delivering sustained high-flow positive air pressure. These systems are much more susceptible to air leaks than is endotracheal intubation. For the purpose of the intensive care unit (ICU), the oronasal mask confers the greatest physiologic improvements with the least amount of airflow resistance and of air leakage from the mouth.
5,
6,
7,
8 and
9 This mask is secured to the patient’s face by the use of adjustable Velcro straps.
NIV can be delivered via the standard type of ventilator found in most ICUs or by a portable ventilator. CPAP is a mode that is often used for patients with acute respiratory failure because of cardiogenic pulmonary edema. BPAP ventilators are capable of delivering very high flow rates and are best suited for use in critically ill patients with concomitant hypoxemia and hypercarbia. They have built-in oxygen blenders and more sophisticated monitoring capabilities and alarm functions. Bilevel machines are pressure-limited and allow for selection of separate inspiratory and expiratory pressure targets.
PHYSIOLOGIC EFFECTS OF NIV
In recent years, there has been a great expansion in the use of NIV in patients with respiratory failure caused by cardiogenic pulmonary edema. NIV reduces respiratory muscle work and facilitates a slower and deeper pattern of breathing, thus resulting in increased minute ventilation (MV
E) and alveolar ventilation. NIV has been shown to improve gas exchange, normalize arterial carbon dioxide, increase arterial oxygen, increase pH, increase tidal volume (
VT), and decrease respiratory muscle work in acute and chronic respiratory failure.
10,
11,
12,
13 and
14 It has also been reported to stabilize certain metabolic parameters such as heart rate, respiratory rate, and blood pressure. Details regarding the systemic hemodynamic and cardiovascular effects of positive-pressure ventilation will be discussed in the section
Invasive Mechanical Ventilation.
SELECTING PATIENTS FOR NIV
Selecting a proper patient is critical to the use of NIV. Successful application of NIV may obviate the need for endotracheal intubation in some patients. A trial of NIV is therefore worthwhile in most patients that do not require emergent intubation, assuming that they have no contraindications. Some potential indicators of success with NIV use are listed in
Table 27.3.
The clinician must exclude patients in whom the use of NIV would be unsafe. NIV is an absolute contraindication in patients at risk of imminent respiratory or cardiac arrest or in those who have already suffered so, and these patients should be promptly intubated. Those with unstable conditions such as shock, myocardial ischemia, or life-threatening cardiac arrhythmias should not be managed with NIV. Patients with altered mental status caused by conditions such as meningitis, stroke, or intracranial hemorrhage (with the exception of hypercapnic encephalopathy), and those who are comatose, agitated or uncooperative also cannot undergo NIV. Ideally, patients being considered for NIV should be able to manage their secretions and those with copious amounts should be considered for intubation. Facial surgery, trauma, or deformities that may prevent adequate fitting of the mask interface, and patients with gastric distension and vomiting are relative contraindications.
NIV IN ACUTE CARDIOGENIC PULMONARY EDEMA
Cardiogenic pulmonary edema and COPD exacerbation are the two acute disorders for which NIV has proven beneficial. In acute cardiogenic pulmonary edema, both CPAP and BPAP have been shown to reduce the work of breathing and intubation rates.
CPAP rapidly improves oxygenation by reexpanding flooding alveoli, increasing functional residual capacity, and
positioning the lung more favorably on its compliance curve.
15 These effects lead to a reduced work of breathing and improved cardiac performance.
15,
16 and
17 A number of randomized, controlled studies have shown that CPAP is effective in treating acute pulmonary edema. Rasanen et al.
18 demonstrated rapid improvement in oxygenation and respiratory rate compared to conventional face mask. Lin and Chiang
19 showed a significantly reduced rate of intubation as compared to standard oxygen therapy, but no difference in mortality. Bersten et al.
20 and Lin et al.
21 subsequently showed similar evidence, however, the latter did show a trend toward improved in-hospital mortality.
Several uncontrolled trials support the use of BPAP for patients with acute pulmonary edema by demonstrating low intubation and complication rates.
22,
23,
24,
25 and
26 However, one of these studies noted a high mortality rate in patients experiencing acute myocardial infarction and cautioned using BPAP in these patients.
26 Evidence produced by Masip et al.
27 in a randomized trial showed significantly reduced intubation rates in patients treated with BPAP compared to standard oxygen therapy. A randomized trial comparing the use of CPAP to BPAP in the treatment of patients with acute pulmonary edema showed significantly more rapid reductions in respiratory rate, dyspnea score, and hypercapnia in the BPAP group compared to the CPAP group, although the trial was aborted because of a greater myocardial infarction rate in the BPAP group.
28 This had been attributed to unequal randomization, however, the results still raise concern for the safety of ventilatory techniques used to treat pulmonary edema complicated by cardiac ischemia or myocardial infarction. A study by Nava et al.
29 did not confirm the increased risk of myocardial ischemia with the use of BPAP as seen in prior studies. However, patients with concomitant hypercapnia experienced more rapid relief of respiratory distress, better gas exchange, and reduced intubation rates, whereas mortality was not affected. A more recent trial compared standard oxygen therapy with NIV (CPAP or BPAP) and found no significant difference in 7- or 30-day mortality between the two groups.
30 In addition, no difference in intubation rate or mortality was seen between the CPAP and BPAP groups. However, the NIV group did achieve more rapid improvement in respiratory distress and metabolic disturbances than did oxygen therapy alone.
For patients with acute cardiogenic pulmonary edema who are hemodynamically stable and meet criteria for NIV, we recommend BPAP as the initial ventilatory mode.
INITIATING NONINVASIVE VENTILATION AND MACHINE SETTINGS
Once the patient has been selected to receive a trial of NIV, it should be initiated as soon as possible. Any delays may result in further deterioration of the patient and increase the likelihood of failure. The oronasal mask should be selected for most patients. Parameters that need to be set with the BPAP mode of ventilation include inspiratory positive airway pressure (IPAP), expiratory positive airway pressure (EPAP), fraction of inspired oxygen (FiO2), and a backup respiratory rate.
IPAP is used synonymously with the term pressure support and EPAP is variably labeled as positive end-expiratory pressure (PEEP). Therefore, an IPAP of 15 cm water and an EPAP of 5 cm water are equivalent to a pressure support of 10 cm water and a PEEP of 5 cm water. The initial target of IPAP and EPAP should be selected on the basis of bedside observation and determined primarily by patient tolerance. Although a universal approach to establishing the initial settings for BPAP has not been established, a minimum IPAP of 10 cm water and EPAP of 5 cm water is usually appropriate as giving the patient higher initial pressures may result in early failure. The VT being delivered with this mode depends on the difference between IPAP and EPAP. For example, the VT will be greater while using an IPAP of 15 cm water and an EPAP of 5 cm water (difference of 10 cm water) compared to an IPAP of 10 cm water and EPAP of 5 cm water (difference of 5 cm water). The goal VT to be delivered in most patients is usually between 8 and 10 mL per kilogram of ideal body weight. If the physician desires to give the patient higher VT IPAP can be titrated up. On the other hand, if the goal is to increase PEEP, EPAP can be increased.
The BPAP machine is a pressure-limited device that supports a spontaneous mode of ventilation. That is, the breath will be delivered only if there is a recognized patient effort. If the physician wishes to add a mandatory minimum machinedirected breath, he can set a backup rate of 10 to 12 breaths per minute. However, these forced breaths are commonly not synchronized with the patient’s effort.
The initial FiO
2 setting should be decided according to the nature of the patient’s respiratory failure and baseline arterial blood gas. In severe hypoxemia, oxygen concentrations of up to 100% can be delivered. Patients with respiratory muscle fatigue and increased work of breathing without severe hypoxemia may require much lower oxygen concentrations. A practical approach is to set the initial FiO
2 to 100%. Arterial blood gas analysis should then be performed after 1 to 2 hours of initiation of BPAP, and the FiO
2 can be titrated down if the PaO
2 is at an acceptable level. It is then reasonable to further titrate down the FiO
2 in small decrements based on the patient’s oxygen saturation on pulse oximetry (usually to a goal greater than or equal to 90%).
Table 27.4 shows a protocol to guide the initiation of NIV.
MONITORING AND TROUBLESHOOTING
As a general rule, patients that are started on NIV should be monitored in the same way as patients who are mechanically ventilated through an ETT. Patients should be observed closely for the first hours after initiation to troubleshoot, provide reassurance, and monitor for deterioration. If there is no stabilization or improvement over this time, NIV should be considered a failure and the patient should be promptly intubated. If the patient is clearly failing immediately after initiation, the clinician should not wait but should proceed to intubation. Some clinical signs that suggest failure include worsening gas exchange, worsening encephalopathy or agitation, inability to clear secretions, inability to tolerate any of the mask interfaces, persistent signs and symptoms of respiratory fatigue, and hemodynamic instability. Reductions in respiratory rate and accessory muscle use, coupled with improvement in thoracoabdominal synchrony and gas exchange suggest a favorable response and good prognosis if seen within 2 hours.
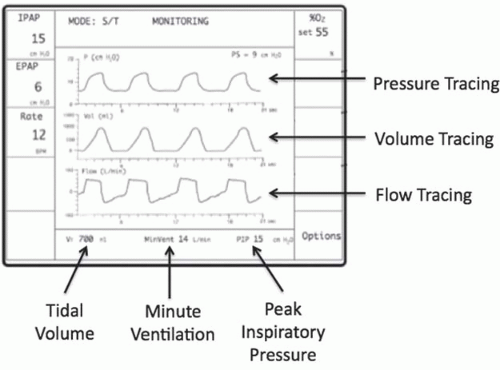
Many patients cannot tolerate BPAP, and this is usually because of patient-ventilator asynchrony (when the phases of inspiration and expiration do not match that of the patient). This may be related to the mode of ventilation being used; however, this should not be the only consideration as the possibility of a mask leak is fairly common and should always be investigated. Waveform displays of breath-by-breath delivered flow, volume, and pressure are available on most ICU ventilators (
Figure 27.1). If the alarms for these parameters are triggered (i.e., low
VT or pressures), this is most likely because of a mask leak. Patients can often be satisfactorily ventilated despite such leakage as most pressure-limited ventilators are able to compensate for leaks by sustaining airflow to maintain mask pressure. If the leak is sufficient enough to interfere with ventilatory support, readjusting the position of the mask on the face with tightening of the Velcro straps may help. In addition, the mask itself may have to be upsized or downsized to ensure a proper fit. Air leaks may also be present if ventilator tubing becomes disconnected from the ventilator or face mask, or if nebulization ports remain open to the atmosphere.
In addition to addressing the possibility of air leaks, asynchrony may also be seen if nonoptimal pressures are being delivered. Increasing or decreasing IPAP can adjust for inadequate VT. PEEP can be adjusted by increasing or decreasing the EPAP. Extreme tachypnea, agitation, and anxiety may be alleviated by the conservative use of sedative agents, such as opiates or benzodiazepines.
Some of the other problems that may be encountered with NIV are nasal congestion or dryness, nasal bridge redness or ulceration produced by excessive mask tension, irritation of the eyes causing conjunctivitis, and gastric insufflation leading to distension or flatulence. Simple efforts such as the use of inline heated humidification, nasal saline sprays, the application of foam rubber spacers or mask pillows, reduction in inflation pressures, or the addition of oral simethicone may help alleviate these problems. Major complications such as aspiration, hypotension, and pneumothorax are seldom seen, and these events can be prevented by carefully selecting patients for NIV.
PATIENTS WITH DO NOT INTUBATE ORDER
NIV is frequently used in patients who have a directive for no intubation but have a potentially reversible cause of respiratory failure such as cardiogenic pulmonary edema. These patients may be good candidates for NIV as their short-term prognosis may be significantly improved. In contrast, NIV is also initiated in patients with advanced stage diseases who have poor prognosis. It is a life support measure and its use should be guided by the goals of care, as it may still offer benefits in alleviating respiratory distress or suffering. More information regarding this topic can be found in the
chapter 31 on
End of Life Care in the CCU.
INVASIVE MECHANICAL VENTILATION
Invasive mechanical ventilation is usually initiated in the CCU for patients with respiratory failure, cardiogenic shock, and
cardiac arrest. If a patient with pulmonary edema has a contraindication to or fails NIV, he should be promptly intubated.
UNDERSTANDING THE MECHANICAL VENTILATOR
A basic understanding of the mechanism of ventilation is useful. The term cycling or control refers to the means by which the ventilator determines that the inspired breath is complete. It is the signal that stops inspiration. This can be sensed either by volume (inspiration stops once the target volume is delivered), pressure (inspiration stops once the target pressure is reached), flow (when flow decreases to a given level, inspiration is terminated), or time (inspiration stops after a preset time interval). The term limit refers to a factor that limits the rate at which gas flows into the lungs and causes inspiration to end before cycling is complete. A limit can be pressure, volume, or flow. Triggering is the signal that opens the inspiratory valve, allowing air to flow into the patient. To initiate a breath, the ventilator must recognize that a set value has been reached. The trigger can be set to time, volume, pressure, or flow. For example, when a patient’s effort reaches the preset pressure value of 1 to 5 cm of water, the machine will deliver a breath.
Breath types can be classified in several different ways. If the patient determines the beginning, duration, and end of a breath, it is said to be spontaneous. If the ventilator controls any of these aspects, the breath is considered to be either assisted or mandatory (controlled). VT is the amount of air delivered with each breath. The normal VT in a 70-kg person is roughly 500 mL. MVE is the product of VT and the respiratory rate, and is the amount of air inhaled or exhaled in 1 minute. The normal MVE is 5 to 8 L per minute. Fraction of inspired oxygen (FiO2) is the percentage of oxygen in the inspired air, and can range from 21% (room air) to 100%. Positive end-expiratory pressure (PEEP) is the pressure set on the ventilator to prevent expiratory alveolar collapse.
MODES OF INVASIVE POSITIVE-PRESSURE VENTILATION
The selection of ventilator mode for a critically ill patient is generally based on the experience of the clinician and institutional preference.
31,
32 and
33 There is little evidence that any particular mode affects clinical outcome.
All basic modes of mechanical ventilation are either volume controlled or pressure controlled.
34 In
Volume control (
volume preset or
volume cycled) ventilation, the machine delivers a preset volume determined by the physician and, within limits, delivers that volume irrespective of the pressure generated within the system. The amount of pressure necessary to deliver this volume will fluctuate from breath to breath based on the resistance and compliance of the patient and ventilator circuit. If the
VT is set at 500 mL, the ventilator will continue to inspire gas until it reaches this goal. Upon completion of the inspired volume, the ventilator will open a valve allowing the patient to passively exhale. It is therefore referred to as
volume-controlled,
pressure variable ventilation.