Chapter 22 Mechanical Cardiac Support
INTRAAORTIC BALLOON COUNTERPULSATION
Intraaortic balloon counterpulsation (IABC) is indicated for the short-term support of patients with severe myocardial ischemia or left ventricular systolic failure. Occasionally, the device may be used for longer than a week, but the incidence of complications increases when use extends beyond 48 hours.1 The insertion and removal of and the complications involved with intraaortic balloon pumps (catheters; IABPs) are described in Chapter 40.
Equipment and Physiology
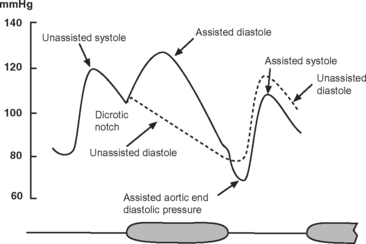
The IABP has two lumens: one for the inflation and deflation of the balloon with helium and one for the measurement of aortic pressure. The catheter is connected to an external console that has a pneumatic pump, a microprocessor for timing inflation and deflation, and a screen that continuously displays the electrocardiogram (ECG), arterial blood pressure, and balloon pressure. The ECG is obtained from electrodes attached to the patient or by “slaving” the ECG from the patient monitor. The balloon is inflated during diastole and deflated just prior to ventricular ejection (Fig. 22-1).
The effects of IABC are twofold. First, because the majority of coronary blood flow to the left ventricle occurs in diastole, balloon inflation at this time increases diastolic aortic pressure and augments coronary perfusion, increasing myocardial oxygen delivery. Second, by deflating just prior to ventricular ejection, aortic end-diastolic pressure is decreased, which leads to a reduction in left ventricular systolic wall tension (and therefore afterload). This augments cardiac output and reduces myocardial oxygen consumption. The diastolic pressure-time index (the area under the diastolic aortic pressure-time curve), which reflects oxygen supply, increases and the systolic time-tension index (the area under the systolic aortic pressure-time curve), which reflects oxygen consumption, is reduced. Thus, IABC improves the balance between myocardial oxygen demand and supply, improves cardiac performance, and reduces left atrial pressure (Table 22-1).
Table 22-1 Physiologic Effects of Intraaortic Balloon Counterpulsation
| Coronary blood flow | ↑ |
| Pulmonary capillary wedge pressure | ↓ |
| Mean diastolic pressure | ↑ |
| Mean systolic pressure | ↓ |
| Mean arterial pressure | ↓ |
| Stroke volume and cardiac output | ↓ |
| Left ventricular afterload | ↓ |
| Heart rate | ↓ |
Indications
The main indications for IABC are listed in Table 22-2.2
Table 22-2 Principal Indications for Intraaortic Balloon Counterpulsation
| Cardiogenic shock | 27.3% |
| Support for high-risk cardiac catheterization | 27.2% |
| Mechanical complications of myocardial infarction | 11.7% |
| Preoperative support for high-risk cardiac surgery | 11.2% |
| Postmyocardial infarction unstable angina | 10.0% |
| Weaning from cardiopulmonary bypass | 4.8% |
| Refractory left ventricular failure | 4.5% |
| Refractory ventricular arrhythmias | 1.3% |
| Cardiac support during noncardiac surgery | 0.5% |
| Other indication or not recorded | 1.5% |
From Stone GW, Ohman EM, Miller MF, et al: Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocar dial infarction: the benchmark registry. J Am Coll Cardiol 41:1940-1945, 2003.
Cardiac Surgery
IABC is widely used to assist weaning from cardiopulmonary bypass (CPB) and to treat hemodynamic instability after surgery (see Chapter 20). IABC may be used after all types of cardiac surgery, but the greatest benefit appears to be in patients undergoing isolated coronary artery bypass graft (CABG) surgery or cardiac transplantation.3 For high-risk patients undergoing CABG surgery, placement of an IABP 1 to 2 hours prior to surgery is associated with reduced mortality rates and shorter stays in the intensive care unit (ICU) and in the hospital compared with patients with undergoing postoperative insertion of an IABP.4–7 Preoperative insertion of an IABP is recommended in patients undergoing CABG surgery who have acute ischemia or an ejection fraction less than 25%.8 Preoperative insertion should also be considered for patients with hemodynamic instability or pulmonary edema secondary to acute mitral valve dysfunction and ventricular septal rupture.
Cardiogenic Shock Secondary to Myocardial Infarction
Cardiogenic shock due to predominant left ventricular failure complicating myocardial infarction is a widely accepted indication for IABC, but there are few data supporting this strategy. Data from the SHOCK trial registry (see Chapter 19) suggest that the use of IABC in combination with revascularization is associated with improved outcome compared to standard medical therapy.9
Myocardial Infarction and Ischemia
IABC is indicated for patients with unstable angina despite maximal medical therapy and for refractory angina following myocardial infarction, even in the absence of hemodynamic instability. Some data show that the routine application of IABC for 24 to 48 hours following successful percutaneous coronary intervention for acute myocardial infarction reduces the incidence of reocclusion of the infarct-related artery and decreases adverse events.10,11 However, this has not been a consistent finding12 and, in the absence of ongoing myocardial ischemia or cardiogenic shock, routine use of IABC after percutaneous coronary intervention is not indicated.
Right Ventricular Dysfunction
IABC is indicated primarily for left ventricular systolic dysfunction, but in patients with right ventricular dysfunction following heart transplantation it produces a substantial improvement in cardiac output and mean arterial pressure and a reduction in central venous pressure (CVP) and pulmonary artery wedge pressure (PAWP).13
Contraindications
Absolute contraindications to IABPs are severe aortic regurgitation, aortic dissection, severe (mobile) atheroma in the descending thoracic aorta, and occlusive vascular disease involving the distal aorta or the femoral or iliac arteries. Minimal diastolic augmentation is obtained in patients with low systemic vascular resistance or excessive diastolic aortic runoff due to systemic-to-pulmonary (e.g., patent ductus arteriosus) or arteriovenous shunts; however, these states are not contraindications. The severity of any dynamic left ventricular outflow tract obstruction will be made worse by IABC because of reduced left ventricular afterload.
IABP Controls
Triggering and Timing
The most useful trigger for balloon deflation is the R wave of the ECG because this signal is usually reliably identified by the device and is coincident with the onset of ventricular systole (see Fig. 1-3). If ECG triggering is unreliable due to variable R wave amplitude or artifact, pressure triggering can be used. Both balloon inflation and deflation are set relative to the upstroke of the arterial pressure waveform. Every minute pumping is suspended for one beat to permit the IABP to confirm appropriate inflation and deflation timing. Optimal timing of deflation results in a 10 to 15 mmHg reduction in aortic end-diastolic pressure (see Fig. 22-1), and manual adjustment of the deflation control may be required to achieve this.
Unlike deflation, in earlier model IABPs, balloon inflation usually has to be set manually. The inflation control is adjusted so that there is a sharp V at the dicrotic notch of the arterial pressure waveform (see Fig. 22-1). Current generation IABPs have an automatic mode in which the machine automatically sets the timing of inflation and deflation. Early IABPs were unable to augment effectively at high heart rates because of limitations on gas shuttling speed, but this is not an issue in current models.
Patient Management During IABC
IABC can result in thrombocytopenia,14 and the platelet count should be monitored daily. Arterial thrombosis can occur but this risk is minimized by ensuring that the balloon is kept cycling at all times.15 Some authors16,17 recommend anticoagulation with unfractionated heparin for patients receiving IABC; however, in a recent randomized trial, this strategy was not shown to result in reduced limb ischemia.18
Timing Errors
Early and Late Inflation
With early inflation (Fig. 22-2), the balloon inflates during late systole, before the aortic valve has closed. The arterial waveform shows augmentation occurring prior to the dicrotic notch. Early inflation results in increased left ventricular wall stress and myocardial oxygen consumption and may result in increased left ventricular end-diastolic volume and pressure. Aortic regurgitation is made worse by early inflation.
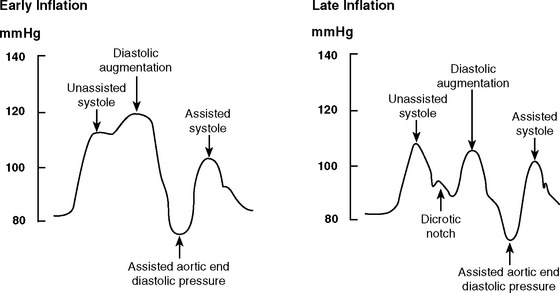
Figure 22.2 Intraaortic balloon counterpulsation showing early and late balloon inflation.
(Image courtesy of Datascope, Fairfield, NJ.)
With late inflation (see Fig. 22-2), the balloon inflates after closure of the aortic valve. The arterial waveform shows diastolic augmentation commencing after the dicrotic notch, absence of a sharp V at the dicrotic notch, and reduced diastolic augmentation. Augmentation of coronary perfusion is suboptimal.
Early and Late Deflation
With early deflation (Fig. 22-3), the arterial waveform shows a pronounced drop in pressure before late diastole. The assisted aortic end-diastolic pressure may be equal to or less than the unassisted aortic enddiastolic pressure, and the assisted systolic pressure may increase. Augmentation of coronary perfusion and afterload reduction are suboptimal. Also, there is the potential for retrograde coronary (and carotid) blood flow, which may exacerbate angina. Myocardial oxygen demand may increase.
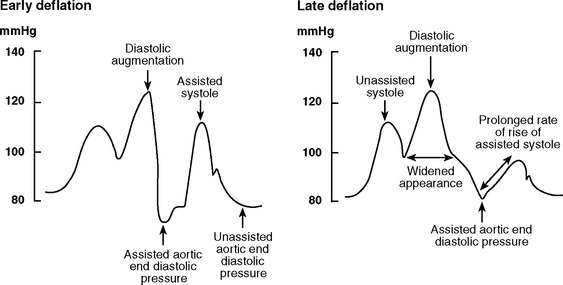
Figure 22.3 Intraaortic balloon counterpulsation showing early and late balloon deflation.
(Image courtesy of Datascope, Fairfield, NJ.)
With late deflation (see Fig. 22-3), the arterial waveform shows an assisted aortic end-diastolic pressure that is equal to or greater than the unassisted aortic end-diastolic pressure. The rate of rise of assisted systole is prolonged and diastolic augmentation may appear widened. Afterload is increased and myocardial oxygen consumption is increased.
Late deflation and early inflation are more deleterious than early deflation and late inflation. Accurate timing, and therefore effective augmentation, are difficult to achieve with tachyarrhythmias or when the dicrotic notch is poorly defined. When the dicrotic notch is difficult to discern, inflation should err on the side of lateness to minimize the risk for increasing afterload and myocardial oxygen consumption. Atrial fibrillation, particularly when rapid, can create problems with triggering and timing and can result in suboptimal augmentation.
Troubleshooting
Poor Augmentation
Failure to achieve optimal diastolic augmentation may be caused by a timing error (see previous material), by incorrect positioning of the IABP catheter, or by incomplete unfurling of the balloon. If technical problems have been excluded, the most likely reason for poor diastolic augmentation is low systemic vascular resistance. High compliance of the arterial tree—which is common in young patients—also reduces the effectiveness of IABC.19,20
Loss of Triggering
Loss of the trigger occurs when the device cannot identify the R wave on the ECG. Options include:
Balloon Leak
Balloon leak occurs in about 1% of patients.21 IABPs incorporate rapid and slow gas-leak alarms that automatically suspend cycling when a leak is detected. The presence of blood in the IABP gas line also implies balloon rupture. A complete rupture results in a gas embolism of one balloon volume (typically about 40 ml) before the device automatically ceases cycling. This generally does not cause major systemic effects and, fortunately, is extremely rare. If balloon rupture is suspected, the gas line should be clamped to prevent blood from entering the console, and the catheter should be removed immediately. The patient should be given 100% oxygen and placed in a head-down position.
Weaning
Unless complications intercede, a patient can be weaned from an IABC once inotropic drug therapy has been reduced to a low level (see Chapter 20). Ideally, the patient should have a mean arterial pressure of at least 70 mmHg, a cardiac output of at least 2.2 l/min/m2, a PAWP less than 18 mmHg, and minimal arrhythmias.15,22 Weaning from the IABP can occur before or after discontinuation of mechanical ventilation. The main advantage of weaning from the IABP first is that the patient can be placed in a sitting position prior to extubation. Augmentation frequency, rather than augmentation volume, should be weaned to ensure continued motion of the balloon and thereby minimize the risk of thrombus formation. In most circumstances, if the patient is stable after 2 to 4 hours of 1:2 augmentation, the IABP can be safely removed. However, if the hemodynamic state is marginal with 1:2 augmentation or the patient is recovering from severe ventricular dysfunction, a trial of 1:3 augmentation over several hours is appropriate before deciding whether to proceed with removal.
Outcome
IABC is employed in high-risk patient populations. Overall mortality rates in large series are about 20%,2,21 with lower mortality rates occurring in cardiac surgery patients and higher rates in patients with cardiogenic shock. Mortality rates directly related to the device itself are very low (0.05% to 0.4%).17,21,23
VENTRICULAR ASSIST DEVICES
The most appropriate candidates for insertion of mechanical ventricular assist devices (VADs) are patients facing imminent death due to acute or chronic heart failure who have preserved function of other organs.24 These patients’ illnesses fall into one of four categories: (1) cardiogenic shock following cardiac surgery; (2) cardiogenic shock following acute myocardial infarction; (3) acute heart failure, usually resulting from myocarditis but also resulting from other causes, such as cardiomyopathy and drug overdose; (4) decompensated chronic heart failure. In each of these categories a VAD may be used as a bridge to recovery, as a bridge to heart transplantation, or as permanent support (destination therapy). In the first three categories, the ideal goal is bridging to recovery. Should recovery not occur, the goal becomes bridging to transplantation. The last group of patients, those with chronic heart failure, usually can be bridged to transplantation, or they may receive a VAD as destination therapy.
The main use of VADs is as a bridge to recovery or transplantation. The use of VADs for destination therapy is, as yet, relatively uncommon. The availability of destination therapy also depends on whether a local program with funding exists. In the United States there are about 5 million people with heart failure, of whom approximately 400,000 have New York Heart Association class IV symptoms, and about 100,000 are younger than 65 years of age. There are approximately 2500 donors per year, making heart transplantation an epidemiologically trivial therapy for heart failure. As a result, there is considerable interest in VADs as destination therapy.25
Indications
Cardiogenic Shock Following Cardiac Surgery
Cardiac failure requiring VAD support occurs in less than 1% of all cardiac operations, as a result of ventricular dysfunction or intractable arrhythmias. Myocardial stunning is a major contributor to postcardiac surgery cardiogenic shock. Recovery from stunning usually occurs within 48 to 72 hours and is unlikely after 5 to 7 days.26 The likelihood of recovery is less if there was poor ventricular function before surgery, if there has been a large perioperative myocardial infarction, or if there are residual lesions (e.g., valve dysfunction, myocardial ischemia). Between 20% and 40% of patients who require a VAD following cardiac surgery survive to hospital discharge. Survival rates are much lower in patients over 65 years (17%) than in patients under 65 years (36%).27
Cardiogenic Shock Following Myocardial Infarction
Even optimally managed with IABC and revascularization, cardiogenic shock has a mortality rate of at least 40%.28 Between 50% and 60% of patients who receive a VAD in this circumstance are able to be weaned from the device, and 38% are discharged from the hospital.27 Recovery occurs within 5 to 7 days, so a short-term device is suitable for nontransplant candidates and a short- or intermediate-term device for transplant candidates.
Myocarditis and Cardiomyopathy
Fulminant, as opposed to acute, myocarditis is characterized by rapid onset and progression, fever, and severe hemodynamic compromise. If supported, patients tend to recover completely. With acute myocarditis, recovery is less likely and VAD support is more likely to be directed toward transplantation. If explantation is not possible after 3 months of VAD support, recovery is extremely unlikely, and the patient should undergo transplantation.29 When a VAD is required, the survival rate in cases of myocarditis is as high as 70%, with approximately half of survivors requiring transplantation and half recovering without transplantation.27,29,30 A short- or intermediate-term device is appropriate, with conversion to a long-term device if required. Some patients with acute cardiomyopathy, notably women with peripartum cardiomyopathy, also have a high rate of recovery with VAD support.
Decompensated Chronic Heart Failure
Decompensated chronic heart failure is the most common indication for long-term VADs. Most patients are on the heart transplant waiting list, and the aim is to bridge them to transplantation. Bridging to heart transplantation is highly effective; it carries a 60% to 90% 1-year survival rate.31 A VAD does not adversely affect survival after transplantation.32 Survival to transplantation and after transplantation may be better with a VAD than with intravenous inotropes.33 Among patients who decompensate acutely and require VAD support, there is a better survival rate if transplantation is delayed by 2 to 4 weeks to allow for end-organ recovery.34
Only one randomized trial of VAD support for nontransplant patients has been published. In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, patients were randomized to a HeartMate (Thoratec, Pleasanton, CA) or to maximal medical therapy. The 1- and 2-year survival rates were 52% and 23%, respectively, for the VAD group and 25% and 8% for the medical therapy group.35 However, the VAD patients had 2.5 times more adverse events, the predominant ones being infection, bleeding, and device malfunction. Infection accounted for 41% of the deaths of the patients who had VADs. At 2 years, the probability of device malfunction was 35%. The incidence of bleeding complications and stroke were 42% and 16%, respectively. Thus, although the treatment effect was three times better than that of medical therapy, there were considerable quality-of-life limitations. Currently, the RELIANT trial is recruiting patients to compare the improved Novacor (Worldheart, Oakland, CA) and the HeartMate XVE (Thoratec, Pleasanton, CA) devices in nontransplant patients.
Between 5% and 15% of patients bridged to heart transplantation experience sustained improvement in native heart function such that the VAD can be explanted. Most of these patients have acute heart failure, but recovery is described in chronic heart failure too. Recovery is more likely when the cause is nonischemic and when there is minimal myocardial fibrosis.36,37 Recovery after more than 2 to 3 months of VAD support is exceedingly unlikely.29,37
Patient Evaluation
General Hemodynamic State
If a patient meets the criteria for a VAD, there are two critical questions to be asked: (1) Is there a reasonable chance of recovery? and (2) Is the patient a candidate for heart transplantation? If the answer to both of these questions is no, in the absence of a destination VAD program, mechanical support should not be offered. However, if the patient is a candidate for transplantation, a short-term device might be used initially if there is a good chance for recovery. Otherwise, a medium- or long-term device should be chosen. When there is clinical uncertainty about the potential for recovery or transplant status, a short-term device to bridge to a long-term device (a “bridge-to-bridge” device) may be used; it does not reduce the chances for survival.39 An example would be where there is uncertainty about whether brain damage has occurred following a cardiac arrest and time is required for neurologic assessment. If a destination VAD program is available, this is an option for non-transplant candidates. Assessment also involves review of other cardiac and noncardiac considerations.
Right Ventricular Function
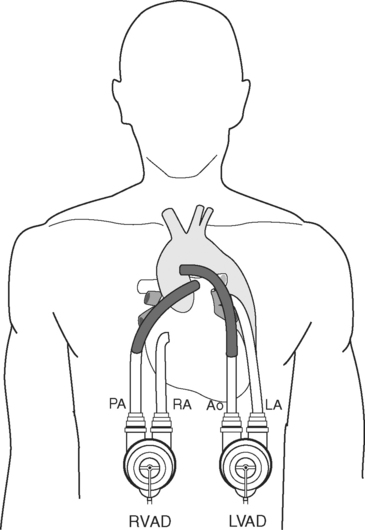
Between 20% and 30% of patients with severe left ventricular failure also have significant right heart dysfunction, but less than 10% of patients receiving an LVAD require an RVAD. Predictors of the need for an RVAD include a CVP more than 16 to 20 mmHg, female gender, nonischemic cause, and preoperative circulatory support.40 High right ventricular volumes and poor contractility (suggested by low pulmonary artery pressures) are also important predictors.40,41 Pulmonary hypertension has been shown by some, but not others, to be a risk factor for requirement of an RVAD.40,41 Most commonly, the need for an RVAD is established during implantation of the LVAD because of low pump flows despite optimal inotropic support. Survival rates are lower when an RVAD is deployed later, so it should be used early if it is indicated.42 The need for an RVAD increases the incidence of bleeding and is associated with a lower survival rate than the use of an LVAD alone.40,43
Intracardiac Shunts
If a patient has a patent foramen ovale or an atrial septal defect, it should be closed during the implant procedure because it may cause significant right-to-left shunting and hypoxemia. This may not be apparent until after the LVAD has commenced and left atrial pressure drops.44 Transesophageal echocardiographic (TEE) assessment specifically looking for atrial shunting should be routinely performed before and after LVAD implantation.
Noncardiac Considerations
In most programs, age limits are the same as those used for heart transplantation (60 to 65 years).25 Age above 65 years is an independent predictor of poor outcome.45 Neurologic function should be fully assessed, especially if there has been a preoperative cardiac arrest or prolonged hypotension. Significant liver dysfunction is a very poor prognostic sign and may contraindicate a VAD. A prothrombin ratio above 1.5 or transaminases or bilirubin more than five times normal were exclusion criteria in the REMATCH trial.35 Severe renal dysfunction is not a contraindication to VAD implantation.46 In the absence of multiple organ dysfunction syndrome (see Chapters 2 and Chapter 20), renal function usually recovers following VAD implantation. Temporary renal replacement therapy is commonly required prior to and following VAD implantation.
Device Selection
Pulsatile Versus Nonpulsatile Pumps
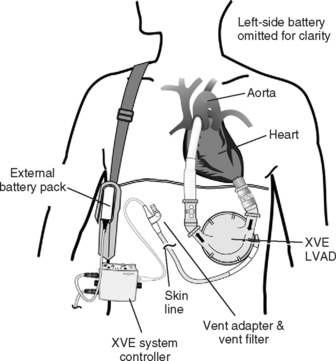
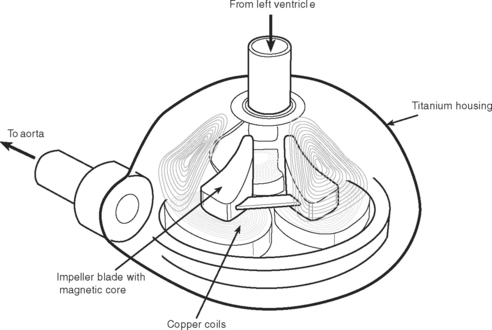
Pulsatile devices (Figs. 22-4 and 22-5) are volumedisplacement pumps. They have a pumping chamber in which blood collects and is then intermittently rapidly compressed, either pneumatically or by an electric motor. Unidirectional blood flow is achieved with valves. They eject a “stroke volume” that is determined by the chamber volume and the outflow resistance. Nonpulsatile (continuous-flow) devices generate flow using either an axial or a centrifugal flow pump (Figs. 22-6 and 22-7). Axial flow pumps are smaller than centrifugal pumps and operate at higher rotational speeds to achieve the same flow and pressure.47
Pulsatile pumps have the longest track record for intermediate- and long-term support. All of the VADs currently approved by the U.S. Federal Drug Administration (FDA) are pulsatile devices. The advantages of pulsatile perfusion are debated. Nonpulsatile flow has been described as causing widespread microcirculatory and functional changes, including changes in the heart, brain, lungs, kidneys, and vascular system. Many of the studies showing these changes are based on short-term studies and are not applicable to long-term support. Animal studies demonstrate that is it possible to provide long-term nonpulsatile flow with no detectable change in end-organ function.48,49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree