Chapter 23 Massive Pulmonary Embolism
Pulmonary embolism usually occurs secondary to venous thrombosis in the deep veins of the lower limbs, proximal to but including the popliteal veins.1 Risk factors for venous thrombosis are listed in Table 23-1.
Table 23-1 Major Risk Factors for the Development of Venous Thromboembolism
| Risk Factor | Examples |
|---|---|
| Venous stasis | Long-distance travel |
| Postoperative recuperation | |
| Plaster immobilization of the lower limb | |
| Pregnancy | |
| Stroke or spinal cord injury | |
| Injury to the vein | Postsurgical (e.g., knee joint surgery) |
| Posttrauma (e.g., fracture of the lower limb) | |
| Prothrombotic states | Antiphospholipid syndrome |
| Factor V Leiden mutation | |
| Other | Malignancy |
| Increasing age |
Massive pulmonary embolism can be defined anatomically as a greater than 50% thrombotic obstruction of the pulmonary vasculature or the occlusion of two or more lobar arteries.2 However, the clinical impact of this obstruction depends on the size of the embolus and on the patient’s underlying cardiopulmonary function. Therefore, it is preferable to define massive pulmonary embolism as that which causes hemodynamic compromise which, by one definition, is a systolic blood pressure of less than 90 mmHg or a drop of 40 mmHg for at least 15 minutes.3 Massive pulmonary embolism constitutes about 4% of all pulmonary embolisms4 and has a mortality rate of 15%.5 Mortality rates increase to 25% in patients with cardiogenic shock and to 65% in patients who require cardiopulmonary resuscitation.5 Submassive pulmonary embolism may be defined as pulmonary embolism in which there is echocardiographic evidence of right ventricular dysfunction in the absence of hypotension.
PATHOPHYSIOLOGY
Because of the high compliance of the pulmonary vascular bed (see Chapter 1), more than 50% of the cross-sectional area must be occluded for pulmonary arterial pressure to rise. The right ventricle is a thin-walled structure that is better suited to volume work than pressure work, and an acute increase in pulmonary vascular resistance sufficient to require a mean pulmonary artery pressure of more than 40 mmHg is likely to cause right ventricular failure and hemodynamic collapse (see Chapter 24). (Patients with chronically elevated pulmonary vascular resistance who have developed right ventricular hypertrophy are able to generate much higher pulmonary arterial pressures.)
Massive pulmonary embolism causes regions of the lungs to be well ventilated but poorly perfused—that is, to have a high ventilation/perfusion (V/Q) ratio. This constitutes dead-space ventilation. From first principles (see Chapter 1), this would be expected to lead to an increased arterial carbon dioxide tension (Paco2) but have a minimal effect on arterial oxygen tension (Pao2; see Chapter 1). However, patients with massive pulmonary embolism are typically hypocarbic and hypoxemic. Low Paco2 is explained by the fact that most patients have marked increase in minute ventilation (secondary to pulmonary irritation and hypoxemia), which offsets the effect of increased alveolar dead space. Hypoxemia results mainly from relative intrapulmonary shunting (i.e., regions of low V/Q ratio). Although pulmonary embolism per se does not cause intrapulmonary shunting, lung units with low V/Q ratios develop rapidly due to the effects of surfactant loss, bronchoconstriction, pulmonary hemorrhage, and pulmonary edema. In addition, the effects of low mixed venous oxygen saturation (caused by low cardiac output), reduced diffusing capacity due to obstruction of the pulmonary capillary bed, and right-to-left shunting across a patent foramen ovale (PFO) also contribute to hypoxemia.
Each lung has a dual blood supply from the pulmonary and bronchial arteries. Thus, even complete obstruction of a lobar branch of the pulmonary artery does not usually cause pulmonary infarction.
DIAGNOSIS
Clinical Findings
The symptoms and signs of pulmonary embolism are variable (Table 23-2), and only a minority of patients have all the classic findings of marked respiratory distress (dyspnea, tachypnea), pleuritic chest pain, and clinical evidence of deep venous thrombosis.
Table 23-2 Presentation of Pulmonary Embolism in Patients with Syncope or Shock
| Signs and Symptoms | Syncope (n = 19) (percentage) | Shock (n = 21) (percentage) |
|---|---|---|
| Tachycardia (heart rate >100/min) | 58 | 86 |
| Tachypnea (breath rate >20/min) | 89 | 81 |
| Dyspnea | 89 | 71 |
| Apprehension | 74 | 71 |
| Accentuated P2 heart sound | 79 | 62 |
| Crackles on chest auscultation | 47 | 48 |
| Fever (temperature >37.5° C) | 21 | 43 |
| Pleuritic chest pain | 63 | 38 |
| Cough | 42 | 33 |
| Coexistent DVT | 42 | 19 |
| Hemoptysis | 5 | 10 |
Adapted from Stein PD, Henry JW: Clinical characteristics of patients with acute pulmonary embolism stratified according to their presenting symptoms. Chest 112:974-979, 1997. DVT, deep venous thrombosis
Electrocardiography
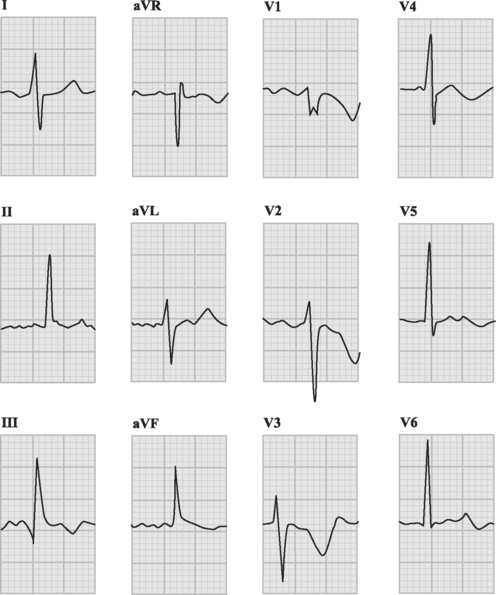
The ECG is usually abnormal but the findings are neither sensitive nor specific for pulmonary embolism. Common abnormalities include sinus tachycardia, anterior (V1 through V4) T wave inversion, partial or complete right bundle branch block, P pulmonale (a tall P wave in lead II, indicative of right atrial enlargement; see Fig. 24-1), and low voltage QRS complexes. The classic findings of an S wave in lead I and a Q wave and T wave inversion in lead III (Fig. 23-1) occur in only 50% of patients.6 T wave inversion in the anterior leads is the most common ECG finding, occurring in 68% of patients with pulmonary embolism overall but in 85% of patients with massive pulmonary embolism.6 The earlier this pattern appears, the greater the size of the embolus. Early normalization of T wave inversion correlates with clinical improvement.
Arterial Blood Gases
Most patients with massive pulmonary embolism have marked hypoxemia, an increased alveolar-arterial Po2 difference (see Chapter 27), and hypocarbia. However, taken in isolation, these blood gas results are neither sensitive nor specific for pulmonary embolism.7 Respiratory alkalosis is common due to hyperventilation, but with massive pulmonary embolism, respiratory and metabolic acidosis can occur.
D-dimer
The product of fibrin cross-linkage, the D-dimer is elevated in any condition in which fibrin cross-links have been cleaved by plasmin. Thus, D-dimer is a nonspecific but highly sensitive test for pulmonary embolism. Several studies have demonstrated its high negative predictive value in pulmonary embolism.8,9 The D-dimer is most commonly used as a screening test in the emergency department, where a normal result virtually excludes pulmonary embolism. Institution of anticoagulation should not be delayed while awaiting this result if there is a high index of clinical suspicion of pulmonary embolism.
Cardiac Biomarkers
Elevated troponin levels (troponin T >0.01 ng/ml) are predictive of a complicated in-hospital course and increased mortality rates in patients with pulmonary embolism.10 High levels of B-type natriuretic peptide (BNP) are also predictive of adverse outcome; a BNP level below 50 pg/ml identifies 95% of patients with benign clinical courses.11 However, the clinical utility of both troponin and BNP in patients with massive pulmonary embolism has yet to be determined.
Scintigraphy
V/Q lung scintigraphy is a noninvasive test for pulmonary embolism that was, until recently, the most commonly performed diagnostic imaging modality. A normal V/Q scan effectively excludes pulmonary embolus. A high-probability scan, as evidenced by multiple segmental mismatches between ventilation and perfusion in the context of a high clinical suspicion, identifies more than 90% of patients with pulmonary embolism.12 However, the utility of V/Q scans is limited by difficulties in interpreting them, especially in patients with underlying lung disease in whom regional variations in ventilation and perfusion confound interpretation of the image. Thus, although agreement among observers is high for normal and high-probability scans, it is substantially lower for intermediate scans, and these are the kinds of scans most commonly encountered in everyday practice. Scans should be performed within 24 hours of clinical suspicion of pulmonary embolus because the changes may revert to normal within a week.
Computed Tomogram Pulmonary Angiography
CTPA has become the imaging modality of choice for diagnosing pulmonary embolism (Fig. 23-2). The technique involves the rapid injection of up to 120 ml of radiocontrast into a peripheral vein using a powered injector. Imaging delay and scan thickness are optimized for the pulmonary arteries in the arterial phase. It is relatively noninvasive, very rapid (imaging is completed easily in one breathhold), and highly accurate. In two studies, the sensitivities for CTPA of four-slice scanners were 90% and 100%, with respective specificities of 98% and 89%.13,14 A negative CT scan reliably rules out pulmonary embolism.15,16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree