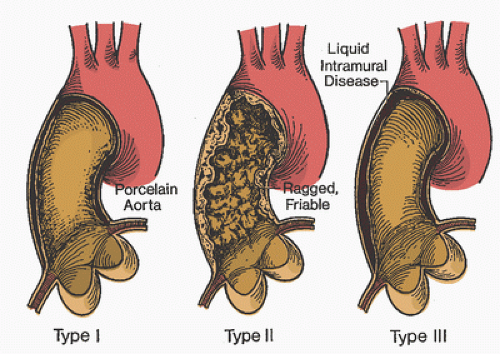
Cryoproteins
Cold agglutinins are serum antibodies that become active at decreased blood temperature and produce agglutination of red blood cells. In some cases, blood cell agglutination may involve complement fixation and lead to significant hemolysis. These antibodies are most commonly directed against antigens on the red blood cells but can also be nonspecific. One very important and most clinically relevant characteristic of cold agglutinins is termed the thermal amplitude, which is the temperature below which the antibodies become activated. As temperature drops below this threshold, antibody activity increases exponentially. In general, this activity reverses as rewarming occurs (
10). Another important parameter of cold agglutinins is termed the titer, which is the concentration of the cold agglutinin expressed as the dilution factor beyond which the agglutination does not occur. A 1:1 titer indicated a relatively low concentration of the cold agglutinin because a 1:1 dilution is sufficient to eliminate the agglutination, while a 1:128 titer indicated a much higher concentration of the cold agglutinin. Clearly, higher cold agglutinin titers (concentrations) are more clinically significant than low titers. There is no widely accepted definition for high versus low titer; however, Lee et al. (
11) suggested titers less than 1:32 as being low and those greater than 1:128 as being high.
The complement system is activated during CPB, especially so during rewarming from hypothermia. Red blood cells agglutinated by cold agglutinins may fix activated complement and
undergo hemolysis, which can be severe, depending largely on the thermal amplitude and titer of the cold agglutinin. For hemolysis to occur, the cold agglutinin and complement activities must overlap. That is, the temperature must be low enough for the cold agglutinins to activate but warm enough for a complement fixation to occur.
Aside from during hypothermic bypass, cold agglutinins seldom produce symptoms because activation most often occurs at temperatures well below the usual range of body temperature. With cold exposure, clinical signs may include acrocyanosis of digits, tip of the nose, or ears from agglutination-induced ischemia. Most commonly, immediate warming of the affected areas reverses the agglutination and thus the ischemia. A patient with prolonged hypothermic CPB would be at risk for multi-organ damage from prolonged vascular occlusion (
12). This is an uncommon but potentially catastrophic consequence of failure to recognize and treat the presence of cold agglutinins.
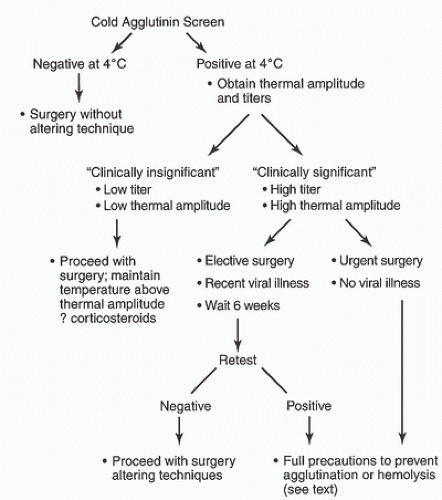
When patients undergo screening for cold agglutinins, a diagnosis can be easily made based on laboratory test results. If screening at 4 °C is negative, no further screening is needed. If the screen is positive at 4°C, the thermal amplitude should be determined and the titer determined for each temperature at which the screen was positive. This will give more precise information for dealing with the cold agglutinin antibodies.
In patients who are not initially screened for cold agglutinins, a diagnosis may be made by astute observation. During hypothermic CPB, agglutination within the vessels may be noted, particularly if the surgeon is wearing magnifying loops. Hemolysis manifested by hemoglobinuria is most often recognized by pink or red-tinged urine. The latter observation, however, still sometimes occurs even in the absence of cryoagglutination. If blood cardioplegia is used, the perfusionist may note agglutination in the cardioplegia delivery system as the blood is cooled (
13,
14). In addition, immediate agglutination of blood in a syringe during phlebotomy may indicate the presence of cold agglutinins. Agglutination also can be confirmed visually by immersing a test tube of blood into an ice slush solution and observing cell clumping on the side of the test tube that often disappears when the tube is warmed. Many cold agglutinins will present during a routine crossmatch done at room temperature. Any of the above findings suggests the presence of clinically significant cold agglutinins, and steps should be taken to prevent adverse reactions during CPB (
13,
15,
16).
Both monoclonal and polyclonal cold agglutinins exist. The monoclonal types usually associated with lymphoreticular neoplasms are generally irreversible. The polyclonal antibodies are often associated with acute infectious diseases such as mycoplasma, infectious mononucleosis, or cytomegalovirus (
12). Production of polyclonal cold agglutinins is typically transient and may remit spontaneously in weeks, but when present it may be associated with acute life-threatening intravascular hemolysis (
12). Leach et al. (
15) developed a comparison of clinically significant and insignificant cold agglutinins (
Table 24.1). An important point in assessing the need to screen for cold agglutinins is that a failure to screen may lead to an adverse outcome, such as myocardial infarction, stroke, or acute renal failure. Without knowledge of the presence of cold agglutinins, these adverse outcomes may be attributed to another cause. For example, hemolysis may be attributed to mechanical trauma to blood from CPB (
17). However, mechanical trauma to blood may be a more important source of clinically significant hemolysis than cold-reacting autoantibodies when the thermal amplitude is less than 22°C.
Treatment of cold agglutinin disease during CPB essentially consists of prevention of complement activation and ultimately of agglutination or hemolysis. Treatment is based on the etiology and the severity of the problem. In a mild case of cold agglutinins (i.e., a case in which there is a very low thermal amplitude, such as 4°C) and/or a low titer of antibodies, minor or no changes in surgical or CPB techniques and, in some cases, treatment with corticosteroids to avoid hemolysis have been advised (
18). For patients with low-titer nonspecific antibodies (and only these patients), Moore et al. (
10) concluded that hypothermic CPB can be performed on these patients without increased risks of hemolytic or agglutination crises; further, minor degrees of hemolysis occur in all patients during hypothermic bypass, especially during the rewarming phase, whether or not cold agglutinins are present.
In a case with clinically significant cold agglutinins (i.e., high thermal amplitude, high titer, or clinical symptoms), a number of changes in surgical technique have resulted in successful surgery using CPB. In the case of clinically significant cold agglutinins caused by acute infection (e.g., a recent viral illness), elective cardiac surgery should be postponed for
several weeks, by which time the antibody may have disappeared (
17). If the urgency of surgery precludes that approach, the most sensible approach is to use either normothermia or mild hypothermia using blood temperatures continuously maintained above the thermal amplitude to avoid the active temperature range of agglutination (
17,
19,
20,
21,
22,
23). Hence, the presence of cryoagglutinins with high titer or high thermal amplitude may represent a reasonable indication for the use of warm cardioplegia myocardial protection techniques while maintaining normothermic systemic temperatures.
In patients with clinically significant cold agglutinins, if it is deemed necessary to use cold cardioplegia, the patient’s blood should be initially flushed out of the coronary circulation with warm crystalloid cardioplegic solution followed by cold cardioplegic solution. Just before removal of the aortic cross-clamp, warm cardioplegic solution should be used to prevent agglutination from blood exposed to a cold heart. Refinements of this latter technique consist of bicaval cannulation with tightening of caval tapes to avoid cooling large amounts of blood in the cardiac and pulmonary circulation. A sump catheter may be placed in the right atrium to retrieve cardioplegic solution until the coronary sinus effluent is clear. In addition, lower-than-normal CPB systemic flows may be used to decrease the noncoronary collateral flow and subsequent cooling of this blood.
If it is uncertain whether this noncoronary collateral flow will cause problems, the heart may be maintained at a temperature above the thermal amplitude (
24). Venting the left ventricle will avoid cooling and stagnation of blood in the left ventricular cavity. Crystalloid cardioplegia has been used rather than blood cardioplegia to avoid agglutination of the cells in the solution when delivered at low temperature (
25). Adjuncts may include a myocardial insulation pad to prevent cooling of blood in structures adjacent to the heart. Using a septal temperature probe in the myocardium to keep the temperature greater than the thermal amplitude may prevent significant activation of cold agglutinins. However, once the red blood cells are flushed out of the coronary circulation, the heart can be cooled to provide myocardial protection provided the above adjuncts are used. In addition, all fluids, blood, plasma, inspired gases, and bolus injections should be warmed (
17) in the periods before and after bypass, especially if the cryoagglutinin has high thermal amplitude.
The literature contains descriptions of successful cardiac surgery after plasmapheresis (
23,
26) or total exchange transfusions in patients with high-titer, high-thermal-amplitude cold agglutinins. There is some evidence that the patient’s own red blood cells may be protected from hemolysis, and if transfusions are required, autologous packed red blood cells may be advantageous (
17). In the case of unexpected agglutination encountered at the time of surgery, several techniques may be useful: verification by the blood bank that cold agglutination is present rather than an unrecognized alloantibody; the use of crystalloid cardioplegic solution to dilute the antibody in the coronary circulation; use of noncardioplegic techniques (e.g., electrical fibrillation); and maintenance of systemic temperatures greater than 28°C to 30°C at which significant amounts of agglutination are unlikely to occur. In addition, the CPB circuit and blood cardioplegia delivery system should be monitored carefully throughout bypass for presence of cell aggregates, and CPB arterial line filters should be used in all cases.
Several cases of fibrin formation and clotting of membrane oxygenators during hypothermic CPB have been reported (
27). Although cold agglutination was initially suspected, none of these patients exhibited agglutinin formation either during preoperative screening or postoperative hematologic workup. No abnormalities in blood coagulation factors VII and VIII or von Willebrand factor were demonstrated in blood samples tested postoperatively, nor were there significant differences between the affected patients’ blood samples and control patients’ blood. However, rapid CPB cooling in conjunction with use of efficient oxygenator heat exchangers having small blood pathways was associated with excessive premembrane CPB line pressure buildup that usually resolved with more moderate cooling strategies or by warming the perfusate.
In summary, all patients undergoing hypothermic CPB should be screened preoperatively for cold agglutinins (
19,
20,
21,
23,
28). If an initial screen is positive, the cold agglutinins should be characterized as to thermal amplitude and titer. Clinical symptoms should also be sought. A patient with low titer, low thermal amplitude, and clinically asymptomatic can tolerate CPB with moderate hypothermia at very low risk with little or no alteration in technique. For clinically significant cold agglutinins with high thermal amplitude and/or titer, if due to a transient viral illness, elective surgery may be postponed for several weeks in the hope that this will decrease cold agglutinins to insignificant levels. However, in severe cases in which the high-titer, high-thermal-amplitude cold agglutinins are not transient, precautions should be taken as outlined above to prevent an agglutination or hemolytic crisis (
Fig. 24.2).