Class I Recommendation
ICD implantation is recommended in patients with CS and one or more of the following:
1. Spontaneous sustained ventricular arrhythmias, including prior cardiac arrest.
2. The LVEF is ≤35 %, despite optimal medical therapy and a period of immunosuppression (if there is active inflammation).
Class IIa Recommendation
ICD implantation can be useful in patients with CS, independent of ventricular function and one or more of the following:
1. An indication for permanent pacemaker implantation.
2. Unexplained syncope or near-syncope, felt to be arrhythmic in etiology.
3. Inducible sustained ventricular arrhythmias (>30 s of monomorphic VT or polymorphic VT) or clinically relevant ventricular fibrillationa.
Class IIb Recommendation
ICD implantation may be considered in patients with LVEF 36–49 % and/or an RV ejection fraction <40 %, despite optimal medical therapy and a period of immunosuppression (if there is active inflammation).
Class III Recommendations
ICD implantation is not recommended in patients with no history of syncope, normal LVEF/RVEF, no LGE on CMR, a negative EP Study and no indication for permanent pacing. However, these patients should be closely followed for deterioration in ventricular function.
ICD implantation is not recommended in patients with one or more of the following:
1. Incessant ventricular arrhythmias.
2. Severe NYHA class IV heart failure.
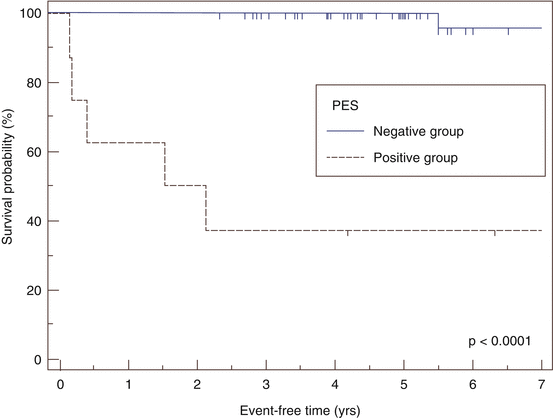
Fig. 12.1
Kaplan-Meier estimation of event free survival as stratified by programmed electrical stimulation by EP study. Vertical markers indicate the time when follow-up was terminated in each patient [20]. PES programmed electrical stimulation
Risk Stratification
While some electrophysiologists will chose to implant a primary prevention ICD in all patients with cardiac sarcoidosis, most agree that further risk stratification is needed to discern who may benefit from the ICD, as some CS patients appear to follow a fairly benign clinical course. In fact, by the most recent expert consensus guidelines, the ICD is contraindicated (Class III recommendation) in patients with no history of syncope, normal ventricular function, no evidence of delayed contrast enhancement on cardiac magnetic resonance imaging (CMRI), a negative electrophysiology (EP) study, and with no indication for pacing [5]. Ultimately, risk stratification may be guided by a combination of CMRI and 18-FDG myocardial positron emission tomography (PET) findings, electrophysiologic testing with programmed electrical stimulation, or by other high risk clinical features such as atrioventricular block, systolic dysfunction, or a history of syncope. Current guidelines recognize the paucity of randomized, prospective data, and cite the limitations of retrospectively analyzed series; ultimately, further research is needed to better advise practice.
Sudden Death Risk Stratification by LV and RV Systolic Function
Due to both the element of active granulomatous inflammation, and the heterogenous involvement of the left ventricle (LV) and/or right ventricle (RV), CS may behave differently than other cardiomyopathies when it comes to risk of ventricular arrhythmias and SCD. Accordingly, a different set of rules may govern SCD risk and ICD indications than the standard LVEF < 35 % that is often used for non-sarcoidosis cardiomyopathies. While CS patients with EF < 35 % should receive a primary prevention ICD in accordance with accepted device guidelines [6], many CS patients with EF > 35 % may also be candidates for primary prevention ICD implantation.
While a lower LVEF is associated with appropriate ICD therapy in several retrospective studies, many patients with only mildly impaired LV function also had substantial risk of ventricular arrhythmias and resultant appropriate ICD shocks and anti-tachycardia pacing [7–9]. In fact, in the study by Kron et al. most primary and secondary prevention patients who received appropriate ICD therapies had an LVEF > 35 %, suggesting that CS patients with mild or moderately reduced LVEF may still be at substantial risk for ventricular arrhythmias [7]. Furthermore, in the study by Betensky et al. 7 of the 17 patients (41 %) with appropriate ICD therapy had LVEF > 35 % [9]. While these studies noted that a number of patients with mild and moderately reduced LVEF received appropriate ICD therapies for VT/VF, Schuller et al. reported that in their primary prevention cohort, no patient with normal left and right ventricular systolic function (LVEF > 50 % and RVEF > 40 %) required ICD therapies over the follow up period [8]. It is from these data that a Class IIa recommendation for consideration of ICD implantation in patients with LVEF 36–49 % and/or RV ejection fraction <40 %, after optimal medical therapy and initiation of immunosuppression if indicated was based.
Sudden Death Risk Stratification with Cardiac Imaging
As discussed in Chaps. 5, 6, and 7, CMRI and 18-FDG myocardial PET appear to be the imaging modalities with the highest sensitivity and specificity in the detection of CS [10–14]. CMRI, in particular, has emerged as the test of choice at many centers in the evaluation of CS, owing in part to a lower false positive rate. CMRI uses T-2 weighted signal and early gadolinium images to detect acute inflammation and late gadolinium enhancement (LGE, also known as delayed contrast enhancement) to assess for fibrosis or scar. Preliminary data also suggest that surveillance with CMRI can assess the efficacy of steroid therapy [15, 16].
Importantly, CMRI has a role in risk stratification and prognosis. In a recent study of 155 consecutive patients with systemic sarcoidosis who underwent CMR for workup of suspected CS, LGE was present in 39 patients (25.5 %), and its presence was associated with a Cox hazard ratio of 31.6 for death, aborted sudden cardiac death, or appropriate ICD discharge [17] over a median 2.6 year follow-up period. Of the twelve patients with sudden cardiac death or appropriate ICD discharge in this study, all had LGE present on CMRI. Sarcoidosis patients without LGE, even those with LV dilatation and severely impaired LVEF, did not experience SCD, suggesting CMRI may provide prognostic data beyond that conferred by ejection fraction alone.
While CMRI may have a higher specificity, 18 F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG PET) appears to detect active inflammation in CS with a slightly higher sensitivity [18, 19]. However, as the uptake of 18F-FDG is seen in other inflammatory myocardial diseases, PET is non-specific for CS and must be interpreted in the appropriate clinical context. Like CMRI, PET carries prognostic value – in a study of 118 patients with suspected CS, 60 % had abnormal cardiac PET findings. Over a median follow-up of 1.5 years, abnormal PET findings were associated with a hazard ratio of 3.9 for death or VT, and an abnormal PET remained a significant predictor of death or VT even after multivariate analysis to adjust for ejection fraction and other clinical variables [13], indicating PET offers prognostic value beyond EF alone.
Sudden Death Risk Stratification with Electrophysiologic Testing
Electrophysiologic study can add useful diagnostic information, particularly if CMRI or PET are inconclusive or unable to be obtained. Electroanatomical mapping, or “voltage-mapping,” of the right ventricle can help identify areas of scar, which can aid in the diagnosis of CS in the right clinical context. There have also been limited studies investigating the role for programmed electrical stimulation (PES) for the inducibility of sustained monomorphic ventricular tachycardia as a means of risk stratification of sudden cardiac death in patients with CS. One such study of 76 patients with CS undergoing PES showed that 6 of 8 patients (75 %) with inducible VT went on to have ventricular arrhythmias and ICD therapies, compared to 1 patient with sudden cardiac death amongst the 68 patients (1.5 %) who had been non-inducible by PES [20]. In another study of 32 patients with CS, 4 of 6 patients (67 %) with inducible VT went on to have appropriate ICD therapies while 2 of 20 patients (10 %) who were non-inducible went on to have ventricular arrhythmias or sudden cardiac death [21]. While these studies suggest a potential role for electrophysiologic testing in risk stratification, these data ultimately need to be replicated in larger cohorts. Furthermore, these studies are limited by mean follow-up periods of 5 years and 2.6 years, respectively; and, in light of CS as a progressive disease, the long-term prognostic value of a single negative EP study is unclear.
Indications for Implantable Cardiac Defibrillator Therapy
The ACC/AHA/HRS 2008 guidelines give a Class IIA recommendation (level of evidence C) for ICD therapy in sarcoidosis patients with evidence of myocardial involvement, regardless of symptoms or presentation [22]. As discussed earlier in this chapter, HRS released an expert consensus statement in 2014 further refining the specific recommendations for ICD therapy as they pertain to patients with CS (Table 12.1) [5]. Class I indications stand for secondary prevention devices and for those with severe LV dysfunction despite a period of optimal medical therapy as per recommendations for patients with other cardiomyopathies [6]. These guidelines also give Class II recommendations for ICD implantation in CS if there are other features considered to be higher risk (e.g., need for permanent pacing, mild to moderately reduced LV (i.e., LVEF 36–49 %) or RV (i.e., RVEF <40 %) systolic function, or inducible VT by EP study.
These recommendations come at a time when practice patterns are heterogenous [23], with some centers tending to implant ICDs in CS patients only with Class I indications (prior ventricular arrhythmias or LVEF ≤ 35 %), while other centers are offering ICDs to all CS patients (in accordance with the IIA recommendation from the 2008 guidelines). And, while the type of patient for which the primary prevention ICD will benefit does remain controversial in CS, what is becoming evident from a number of observational studies is that among those CS patients who do receive ICDs, both appropriate and inappropriate ICD therapies are common.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


