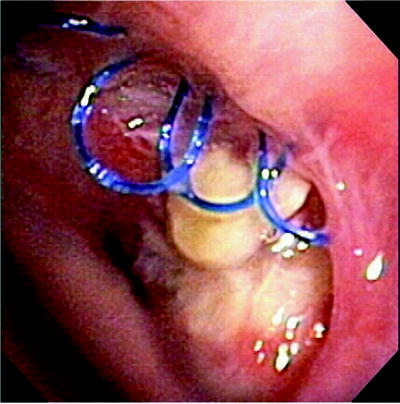
Fig. 45.1
(a) Example of a healthy right anastomosis. (b) Image 1.1b shows a healthy left anastomosis
Anatomy
While normally there is a dual blood supply from the bronchial and the pulmonary arteries, the bronchial circulation is not reestablished in standard lung transplantation. Previous anatomic studies have defined fairly consistent origins of the bronchial arteries from the intercostal arteries and descending thoracic aorta. The bronchial circulation provides an important blood supply to the major airways and supporting structures of the lungs. The bronchial arteries arise from the aorta or the intercostals and travel through the hila where small arterials penetrate the muscular layer of the airway and terminate in the bronchial mucosa. This termination is in the form of a submucosal plexus which eventually results in a collateral circulation between the pulmonary and bronchial circulations. Each proximal main stem bronchus receives its primary blood supply from the bronchial circulation although the pulmonary circulation can contribute via retrograde collaterals. While the much larger pulmonary circulation is reestablished at transplantation, the bronchial circulation is not. Therefore, bronchial viability and anastomotic healing are dependent upon retrograde blood flow from the pulmonary to bronchial circulation leaving the anastomosis and distal airway in jeopardy. Coronary collateral blood supply arises at atrial branches from the left and right coronaries. This collateral supplies the main carina as well as the proximal left and right main stem bronchi explaining why the more proximal airways do not seem to suffer the same ischemic risk.
Risk Factors
The etiology of airway complications is likely multifactorial. Most theories attribute AC to ischemia, infection, or a combination of the two.
Anastomotic Ischemia
Airway complications have long been attributed primarily to ischemia of the donor bronchus in the immediate transplant period. The native lung blood supply is dual with a component coming both from the bronchial and pulmonary systems. In the standard transplantation surgery, the bronchial arteries are severed and not reanastomosed due to their small size. Collaterals take up to 4 weeks to develop, during which time the donor bronchus is completely dependent upon the retrograde flow from the pulmonary artery. Unfortunately, this is a low-flow, low-pressure system that is poorly oxygenated. This is believed to be a major factor that predisposes the donor bronchus to ischemia leading to necrosis, sloughing, infection, dehiscence, and/or stricture. There are additional factors in the postoperative course which may also play a role. Complications such as hypotension, low cardiac output, corticosteroids, glucose levels, poor tissue perfusion, volume status (overload or dehydration), circulating antibodies, and cytokines among others can affect tissue oxygenation at the cellular level.
Donor Bronchus Length
Closely relating to ischemia is the length of the donor bronchus. A donor bronchus with excessive length is likely more susceptible to ischemia due to the lack of collateral flow in the initial posttransplant period. Therefore, a longer bronchus has less blood flow at the anastomosis. For this reason, the donor bronchus should be shortened with the anastomosis being as close as possible to the secondary carina. Although beneficial from an ischemia standpoint, an anastomosis that is in close proximity to the secondary carina may make the management of a complication more difficult. This is particularly true in the case of stenting as there may not be adequate length to seat the distal end of the stent.
Anastomotic Techniques
Every attempt is made to preserve undisturbed peribronchial tissue in an attempt to maintain the microvasculature. The benefits of particular anastomotic techniques are debated among surgeons, and there is no consensus practice. Wrapping the bronchial anastomosis with omentum or another vascularized pedicle has demonstrated reestablishment of collateral circulation at the level of the donor bronchus. Bronchial omentopexy appeared to be important in the early success reported by the Toronto group. The improvement in early neovascularity in addition to separating the bronchus from the mediastinum and pulmonary artery was an attempt to minimize the effect if a dehiscence were to occur. This procedure added complexity and morbidity primarily due to the addition of a laparotomy as well as a diaphragmatic defect. For these reasons, omentopexy is rarely practiced now. Additional anastomotic coverage with peribronchial tissue or other vascularized flaps have also been described although the data is weak as to their benefit. Anastomotic technique varies by institution and surgeon. There is some data to suggest that the risk of airway complication may be related to the type of anastomosis (see Fig. 45.2). The two most common types of anastomosis are the telescoping technique and the end-to-end anastomosis. Data exists which suggests that the telescoping technique is associated with more complications, particularly obstruction. Despite this, some surgeons still perform a telescoping anastomosis but agree that an exaggerated intussusception should be avoided whenever possible. An intussuscepted airway will predispose to an obstruction simply due to its anatomy. An obstruction seen in this airway will also be more difficult to dilate. In addition to this potential mechanical obstruction, any additional amount of sloughed necrotic tissue will lead to earlier obstructive symptoms. A significant intussusception may also allow for entrapment of the microorganisms between bronchi due to the microbiologic environment. For these reasons, most centers have adopted an end-to-end anastomotic technique as the procedure of choice unless donor-recipient bronchial size mismatch exists.
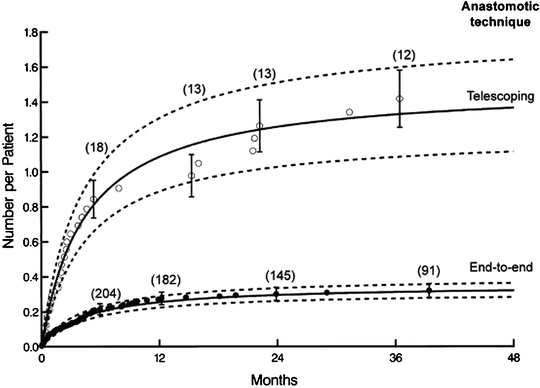

Fig. 45.2
Anastomotic techniques. Shows the impact of airway complications (AC) based on type of anastomosis. The rate of AC increases with a telescoping anastomosis. (Reprinted with permission from Mehta AC, et al. Impact of Anastomotic Airway Complications After Lung Transplantation. Ann Thorac Surg. 2007;84:401–409, Graph 4.)
Donor/Recipient Size Mismatch
Airway complications also appear to be related to donor and recipient size mismatching. Differing diameters of donor and recipient bronchi have traditionally been treated with a telescoped anastomosis. This telescoping may lead to unwanted and potential deleterious stress at the suture line. Almost always, the size mismatch is a donor airway that is smaller than the recipient. A technique to manage this size mismatch is to perform an upper lobectomy on the recipient and anastomose the donor main stem to the bronchus intermedius on the right or the left lower lobe airway on the left.
Miscellaneous Factors
Several other unavoidable risk factors which predispose a lung transplant anastomosis to complications exist. Although many of these risk factors may appear to be logical, most have not been demonstrated across institutions or in multi-institutional studies. Factors such as a male donor and a tall donor have been suggested. The anastomosis is seldom tension-free due to the wall stress created by positive pressure ventilation. This stress may predispose to injury and/or dehiscence. The length of donor time on mechanical ventilation has also been suggested with a time of 50–70 h increasing the probability of an airway complication. This may be related to an increased risk of infection while intubated. Airway anastomoses are sutured in the setting of a contaminated field. The necrosis often seen also provides a nidus for microbiologic growth. This milieu in conjunction with a suppressed immune system makes infection a major concern.
Immunosuppression/Medications
Although there have been considerable improvements in lung transplant survival, there is still a lag behind other solid organ transplants. Lung transplant patients are maintained at higher levels of immune suppression, but despite this, both acute and chronic rejection remain difficult problems to control, and chronic rejection manifested as bronchiolitis obliterans syndrome (BOS) occurs earlier with more dire consequences than other solid organ transplants. Immunosuppression is critical for the survival of an allograft; however, this may predispose to airway complications due to increased susceptibility to infection and diminished healing. Most immunosuppressive regimens consist of a calcineurin inhibitor (typically tacrolimus) in conjunction with an antilymphocyte (mycophenolate mofetil or azathioprine) and corticosteroid. Deserving special mention is sirolimus (rapamycin, Rapamune) which is a novel macrolide originally developed as an antifungal agent. It was later discovered to have potent immunosuppressive and antiproliferative properties which led to its use in renal and later lung transplantation. While the major drawback of calcineurin inhibitors is renal toxicity, this is seen at a much lower incidence with sirolimus. Sirolimus has been shown to be effective in controlling acute rejection and does not have overlapping toxicities with other immunosuppressants without the associated renal toxicities. This combination would make sirolimus appealing as an adjunctive medicine for the lung transplant population. However, in de novo recipients, sirolimus was shown to dramatically increase the rate of catastrophic airway complications. In particular, the rate of dehiscence was found to be unacceptably high in the early transplant period. The current recommendation is avoidance of sirolimus for at least 90 days after transplantation.
Description and Management of Airway Complications
Comparison of airway complications after lung transplantation is made difficult by the lack of universally accepted classification system. However, there are several classification systems which address airway complications which are utilized. (Table 45.1)
Table 45.1
Classification of airway complications
Classification of airway complications | |
|---|---|
Stenosis/stricture | Anastomotic bronchial stenosis |
Stenosis <50% of bronchial diameter | |
Stenosis >50% of bronchial diameter | |
Non-anastomotic bronchial stenosis | |
Stenosis <50% of bronchial diameter | |
Stenosis >50% of bronchial diameter | |
Necrosis and dehiscence | Grade I |
No slough or necrosis | |
Well-healed anastomosis | |
Grade II | |
Any necrotic mucosal slough observed but no bronchial wall necrosis | |
Grade III | |
Bronchial wall necrosis within 2 cm of anastomosis | |
Grade IV | |
Extensive bronchial wall necrosis extending >2 cm from anastomosis | |
Exophytic granulation tissue | Obstructing <50% of the bronchial lumen |
Obstructing >50% of the bronchial lumen | |
Malacia | Diffuse tracheal/bronchial |
Anastomotic location | |
Fistula | Bronchomediastinal fistula |
Bronchopleural fistula | |
Bronchovascular fistula | |
Infectious | Anastomotic infections |
Bacterial | |
Fungal | |
Non-anastomotic infections | |
Bacterial | |
Fungal | |
Viral |
Airway complications can be considered temporally, by etiology or descriptively. Several of the more common descriptive classifications include necrosis, dehiscence, stenosis/stricture, granulation tissue, infection, or fistula. Complications can be categorized as partial or full-thickness necrosis, obstruction, infection, or fistula. Airway necrosis, though not always considered a complication, may cause obstructive symptoms and is the early range of the spectrum including bronchial dehiscence. Obstruction may include airway stenosis from stricture, granulation tissue, or malacia. Included in obstruction is stricture at the anastomosis as well as a non-anastomotic narrowing in segmental or subsegmental airways.
Necrosis
Necrosis is seen at many surveillance bronchoscopies but is not necessarily considered a complication. The incidence of necrosis is unknown as because it is not typically reported. An eschar is frequently seen at the anastomosis as well as in segmental bronchi. Necrosis is usually thought of as part of the healing phase as the airway sloughs the ischemic tissue and is subsequently replaced with healthy mucosa as revascularization occurs (Fig. 45.3). Necrosis is seen early in the healing phase but usually will improve within the first few weeks. An ischemic airway with necrosis may eventually lead to dehiscence.
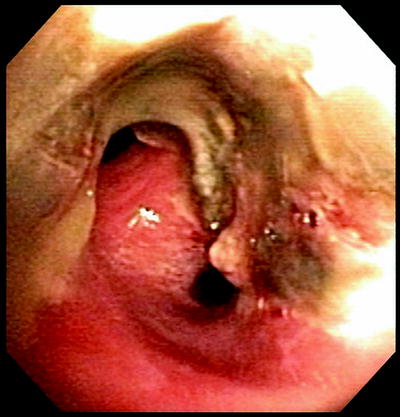

Fig. 45.3
Necrosis image. Necrosis in the immediate transplant period. This is a typical sloughing mucosa seen in the first few weeks after a lung transplant. This is the RUL and RBI seen with necrotic mucosa at 3 weeks posttransplant.This necrosis led a significant stricture, seen below
Airway Dehiscence
Airway dehiscence can be a catastrophic complication, especially in the immediate postoperative period. This is typically thought of on a continuum with necrosis. The inciting event is typically ischemia, though infectious complications may be responsible. These problems are difficult to treat and lead to significant morbidity and mortality (Fig. 45.4). Dehiscence can arise despite meticulous suturing technique, wrapping of the anastomosis, and in the setting of a previously uneventful postoperative course.
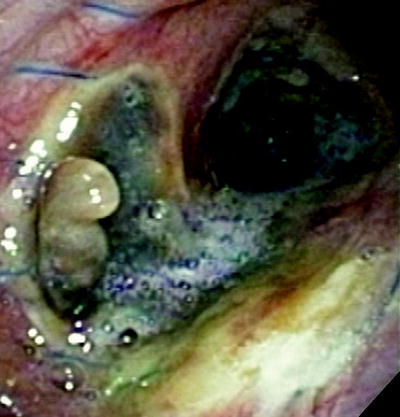

Fig. 45.4
Dehiscence. A dehiscence is seen along the medial and posterior wall 2 weeks after transplantation. The dehiscence is seen in the right main stem bronchus. This was successfully managed with a SEMS
A dehiscence must be considered when forming a differential diagnosis in any lung transplant patient having a difficult course, especially in one with a prolonged pneumothorax or pneumomediastinum. It can be difficult to diagnose as many of the signs or symptoms which may point toward a dehiscence are those that are commonly seen in the posttransplant period. A chest roentgenogram may intimate dehiscence by pneumomediastinum or pneumothorax while a chest CT has a higher degree of sensitivity and specificity. A CT scan may show dehiscence as evidenced by peribronchial air. The gold standard for diagnosis is bronchoscopy. In the setting of severe necrosis, it may be difficult to see the actual site of dehiscence; however, there are several clues seen endoscopically. Significant necrosis and loose sutures can be a clue that the anastomosis is at risk. Figure 45.5 shows an example of necrosis and loose sutures in an airway which eventually dehisced. Ischemia of the bronchial wall, tension at the anastomosis, positive pressure ventilation, and infection are all considered to be risk factors for dehiscence. Four grades of dehiscence have been described and are included in Table 45.1.
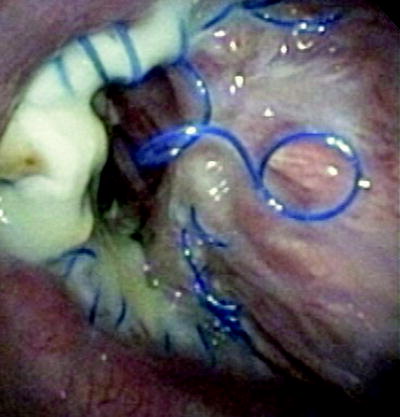

Fig. 45.5
Loose sutures necrosis. This is an example of loose sutures and necrosis in an airway which eventually dehisced
Management of dehiscence depends on the severity. Grade 1 and 2 bronchial dehiscence requires no intervention except close monitoring. Grade 3 and 4 bronchial dehiscence can be devastating complications. Dehiscence has been managed successfully surgically; however, this is fraught with many complications and a high mortality, primarily owing to the critical respiratory condition of the patient at the time of surgery. Cyanoacrylate glue has been successfully used to close a dehiscence, although this technique will not be successful most of the time. A more conventional approach has been described using uncovered self-expandable metallic stents (SEMS). In this technique, granulation tissue, one of the commonly described complications caused by self-expanding metal stents, is used as a benefit. After the airway patency is optimized with gentle dilation and/or debulking, a non-covered self-expandable metallic stent (SEMS) is deployed across the dehiscence and closely followed. As soon as epithelialization/granulation tissue develops, typically within 1–3 weeks, the stent is removed and replaced until the visible defect is closed. Several cycles of this technique have been shown to allow the bronchial wall to heal. An example of this technique is shown in Fig. 45.6a–e. This technique requires expertise in placing and more importantly in removing SEMS as overly aggressive removal may worsen the injury, often with catastrophic consequences. Bronchial dehiscence, even after successful management allowing for closure, may leave behind other complications such as stenosis, bronchomalacia, exophytic granulation tissue, or fistula formation.
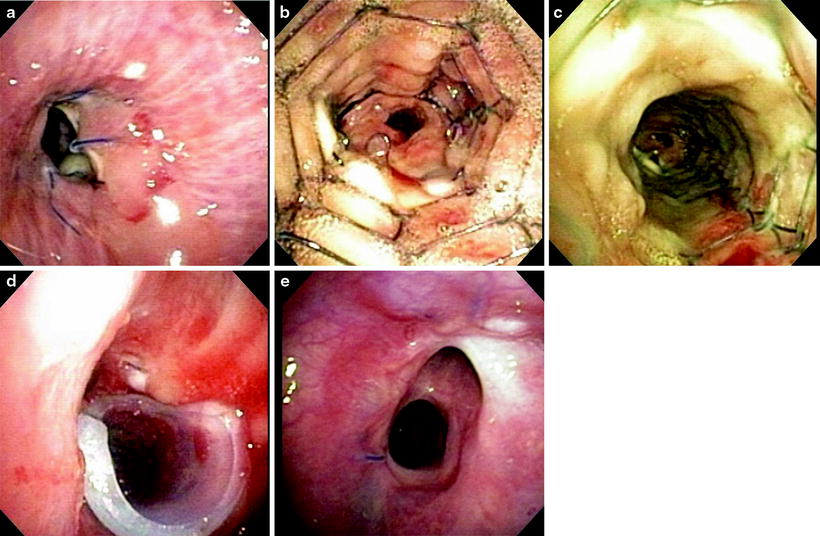

Fig. 45.6
(a) Dehiscence. The arrow pointing to the opening seen is actually a full-thickness dehiscence into the mediastinum. The RBI and RUL were completely occluded by the medial wall. This was treated with SEMS placement and removal × 2 followed by a silicone stent with excellent results, both in appearance and clinical outcome. (b) Placement of a SEMS across the area of dehiscence. This will allow for granulation tissue to develop and initiate the healing process. The non-covered stent was chosen both for its increased ability for inducing granulation tissue and to allow for drainage of the right upper lobe (RUL) as the stent “jails” the orifice. (c) Two weeks after placement of the SEMS, there is granulation forming along the stent. Once the granulation forms, the stent should be removed and replaced. (d) After placement and removal of a SEMS and silicone, stent was required for both stricture and a component of malacia. The silicone stent was also customized by “notching” an area to allow for RUL drainage. The arrow is pointing to the RUL. (e) The airway has healed, and the patient has a normal level of functioning
Granulation Tissue
Exophytic granulation tissue may be seen as a result of an exaggerated immune response, similar to a keloid reaction. This is likely related to an exuberant healing response leading to an influx of inflammatory mediators and immune cells. A reaction to loose sutures in the airway or an infection may also be responsible (Fig. 45.7). This is a response seen at the anastomotic site, typically within several months. The major complication tends to be obstructive symptoms, either relating to decreased airflow or mucus clearance. Cough, dyspnea, or a drop in airflows measured by spirometry may be the first clues and should be confirmed bronchoscopically.