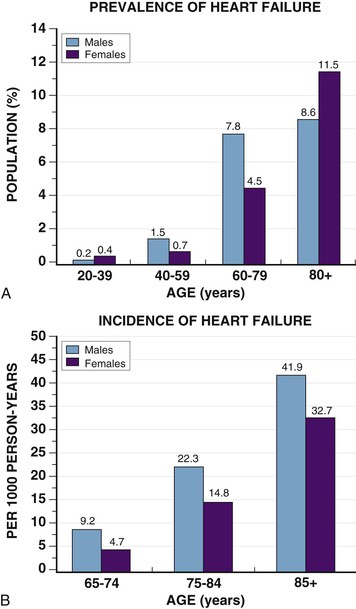
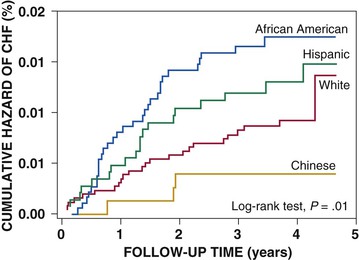
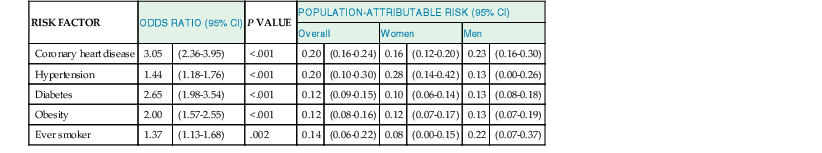
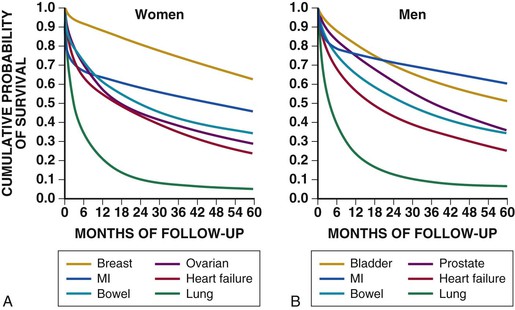
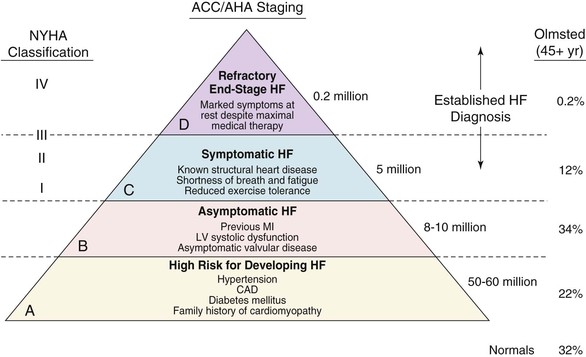
Douglas L. Mann The worldwide prevalence and incidence rates of heart failure (HF) are approaching epidemic proportions, as evidenced by the relentless increase in the number of HF-related hospitalizations, the growing number of HF-attributable deaths, and the spiraling costs associated with the care of patients with HF. Worldwide, HF affects nearly 23 million people. In the United States, the most recent epidemiologic data suggest that 5.1 million Americans 20 years of age and older have HF, and it is estimated that by 2030, the prevalence will increase 25% from current estimates.1 Estimates of the prevalence of symptomatic HF in the general European population are similar to that in the United States, ranging from 0.4 to 2%.2 The prevalence of HF rises exponentially with age, and the condition affects 4% to 8% of people older than 65 years of age (Fig. 25-1A). Although the relative incidence of HF is lower in women than men for all age groups (Fig. 25-1B), women constitute at least half of the cases of HF because of their longer life expectancy, and the overall prevalence of HF is greater in women than in men 80 years of age and older.1 In the ARIC (Atherosclerosis Risk in Communities) study, funded by the National Institutes of Health (NIH), the age-adjusted incidence of HF was greatest in black men, followed by black women, white men, and white women. The higher incidence of HF in blacks was attributed to the greater levels of atherosclerosis risk factors in this population.3 Similar findings were observed in the NIH-funded MESA (Multi-Ethnic Study of Atherosclerosis) study, which showed that blacks had the highest risk for development of HF, followed by Hispanic, white, and Chinese Americans1 (Fig. 25-2). In North America and Europe, the lifetime risk of developing HF is approximately 1 in 5 for a 40-year-old. The overall prevalence of HF is thought to be increasing, in part because current therapies of cardiac disorders, such as myocardial infarction (MI), valvular heart disease, and arrhythmias, are allowing patients to survive longer. Very little is known with respect to the prevalence or risk of developing HF in emerging nations because of the lack of population-based studies in those countries.4 Although HF was once thought to arise primarily in the setting of a depressed left ventricular ejection fraction (LVEF), epidemiologic studies have shown that approximately one half of patients who develop HF have a normal or preserved EF (EF > 50%). Accordingly, patients with HF are now broadly categorized as having either (1) HF with a reduced or depressed EF (HFrEF) of 35% or less, also referred to as systolic failure, or (2) HF with a preserved EF (HFpEF) at 50% or greater, also referred to as diastolic failure. HF in patients with an EF in the range 35% to 50% represents a “gray zone”; such patients are likely to have at least mild systolic dysfunction. The epidemiology of HFpEF is discussed in Chapter 27. Risk factors for the development of HF in men and women include coronary artery disease (CAD), hypertension, diabetes, obesity, and smoking.5 Of importance, the burden of all risk factors among HF cases has increased over time, with significant increases for hypertension, obesity, and smoking. However, the relative contribution of risk factors to the development of HF remains controversial, with some population-based studies suggesting that hypertension has the highest attributable risk and others suggesting that CAD has the greatest impact on the development of HF. In a more recent population-based case-control study, the population-attributable risk for developing HF was greatest for CAD, followed by diabetes, obesity, hypertension, and smoking (Table 25-1). Of interest, sex-based differences in the cause of HF also were noted, with hypertension playing the greatest role in women and coronary disease in men. Although obesity is a risk factor for the development of HF, obese patients with HF seem to enjoy a more favorable clinical prognosis. The association between obesity, a traditional cardiovascular risk factor, and improved clinical outcomes in patients with HF (i.e., reverse epidemiology) has been called the “obesity paradox.” As shown in Table 25-2, any condition that leads to an alteration in left ventricular (LV) structure or function can predispose the patient to the development of HF. Although the etiology of HF in patients with a preserved EF differs from that in patients with depressed EF (see Chapter 27), considerable overlap between the etiologic mechanisms for these two conditions is recognized. In industrialized countries, CAD is the predominant cause in men and women and is responsible for 60% to 75% of cases of HF. Hypertension contributes to the development of HF in a significant number of patients, including most patients with CAD. Both CAD and hypertension interact to augment the risk of HF. Rheumatic heart disease remains a major cause of HF in Africa and Asia, especially in the young. Hypertension is an important cause of HF in the African and African American populations. Chagas’ disease is still a major cause of HF in South America.6 As developing nations undergo socioeconomic development, the epidemiology of HF is becoming similar to that of Western Europe and North America, with CAD emerging as the single most common cause of HF. TABLE 25-2 Etiology of Chronic Heart Failure * Condition(s) that also can lead to HFpEF. In 20% to 30% of the cases of HFrEF, the exact etiologic basis is not known. These patients are referred to as having nonischemic, dilated, or idiopathic cardiomyopathy if the cause is unknown (see Chapter 65). Previous viral infection (Chapter 67) or toxin exposure (e.g., with excess alcohol consumption [Chapter 68] or use of chemotherapeutic agents [Chapter 69]) also may lead to a dilated cardiomyopathy. Although excessive alcohol consumption can promote cardiomyopathy, alcohol consumption per se is not associated with increased risk for HF and may protect against the development of HF when consumed in moderation.7 It also is becoming increasingly clear that a large number of cases of dilated cardiomyopathy are secondary to specific genetic defects, most notably those in the cytoskeleton (see Chapter 65). Most of the forms of familial dilated cardiomyopathy are inherited in autosomal dominant fashion. Mutations of genes encoding cytoskeletal proteins (desmin, cardiac myosin, vinculin) and nuclear membrane proteins (lamin) have been identified thus far. Dilated cardiomyopathy also is associated with Duchenne’s, Becker’s, and limb-girdle muscular dystrophies (see Chapter 87). Conditions that lead to high cardiac output (e.g., arteriovenous fistula, anemia) seldom are responsible for the development of HF in a normal heart. In the presence of underlying structural heart disease, however, such conditions often lead to overt congestive failure. Although several recent reports have suggested that the mortality for patients with HF is subsiding, the overall mortality rate remains higher than for many cancers, including those involving the bladder, breast, uterus, and prostate. In the Framingham Heart Study, the median survival was 1.7 years for men and 3.2 years for women, with only 25% of men and 38% of women surviving 5 years. European studies have confirmed a similar poor long-term prognosis2 (Fig. 25-3). More recent data from the Framingham Heart Study have examined long-term trends in the survival of patients with HF and shown improved survival in both men and women, with an overall decline in mortality of approximately 12 % per decade from 1950 to 1999. Moreover, recent reports from Scotland, Sweden, and the United Kingdom suggested that survival rates after hospital discharge also may be improving.2 Of note, the mortality for HF in epidemiologic studies is substantially higher than that reported in clinical HF trials involving drug and/or device therapies, in which the mortality figures often are deceptively low, because the patients enrolled in trials are younger, are more stable clinically, and tend to be followed more closely clinically. The role of sex in HF prognosis remains a controversial issue with respect to HF outcomes. Nonetheless, the aggregate data suggest that women with HF have a better overall prognosis than do men.1 However, women appear to have a greater degree of functional incapacity for the same degree of LV dysfunction and also have higher prevalence of HF with a normal EF (see Chapter 27). Controversy also has arisen regarding the impact of race on outcome, with higher mortality rates being reported in blacks in some but not all studies. In the United States, HF affects approximately 3% of blacks, whereas in the general population worldwide, the prevalence is approximately 2%.8 Blacks with HF present at an earlier age and have more advanced LV dysfunction and a worse New York Heart Association (NYHA) functional class at the time of diagnosis. Although the reasons for these differences are not known, as noted previously, differences in HF etiology might explain some of these observations. Additional socioeconomic factors also may potentially influence outcomes in black patients, such as geographic location and access to health care. Age is one of the strongest and most consistent predictors of adverse outcome in HF (see later under “Special Populations”).9 Many other factors have been associated with increased mortality in patients with HF (Table 25-3). Most of the factors listed as outcome predictors have survived, at least, univariate analysis, with many standing out independently when multifactorial analysis techniques are used. Nonetheless, it is extraordinarily difficult to determine which prognostic variable is most important to predict individual patient outcome either in clinical trials or, more important, during the day-to-day management of an individual patient. To this end, several multivariate models for predicting the HF prognosis have been developed and validated. One such model is the Seattle Heart Failure Model, derived by retrospectively investigating predictors of survival among patients with HF in clinical trials. The Seattle Heart Failure Model provides an accurate estimate of 1-, 2-, and 3-year survival rates with the use of easily obtained clinical, pharmacologic, device, and laboratory characteristics and is accessible free of charge to all health care providers as an interactive web-based program (http://depts.washington.edu/shfm). The observation that the renin-angiotensin-aldosterone, adrenergic, and inflammatory systems are activated in HF (see Chapter 22) has prompted examination of the relationships between a variety of biochemical measurements and clinical outcomes (see Table 25-3). Strong inverse correlations have been reported between survival and plasma levels of norepinephrine, renin, arginine vasopressin (AVP), aldosterone, atrial and brain natriuretic peptides (ANP and BNP) and N-terminal pro–B-type natriuretic peptide (NT-proBNP), endothelin-1, and inflammatory markers such as tumor necrosis factor (TNF), soluble TNF receptors, C-reactive protein, galactin-3, pentraxin-3, and soluble ST2 (see also Chapter 23). Markers of oxidative stress, such as oxidized low-density lipoprotein and serum uric acid, also have been associated with worsening clinical status and impaired survival in patients with chronic HF. Cardiac troponins T and I, sensitive markers of myocyte damage, may be elevated in patients with nonischemic HF and predict adverse cardiac outcomes. The association between a low hemoglobin or hematocrit and adverse HF outcomes also has long been recognized and recently has garnered considerable attention after several reports illustrated the independent prognostic value of anemia in patients with HF with either reduced or normal ejection fraction.10 Renal insufficiency is associated with poorer outcomes in patients with HF; some uncertainty remains, however, regarding whether renal impairment is a simply a marker for worsening HF or whether renal impairment might be causally linked to worsening HF. Although more common in patients hospitalized for HF, at least some degree of renal impairment is still present in approximately one half of stable outpatients with HF. Patients with renal hypoperfusion or intrinsic renal disease show an impaired response to diuretics and angiotensin-converting enzyme (ACE) inhibitors and are at increased risk for adverse effects during treatment with digitalis. In a recent meta-analysis a majority of patients with HF had some degree of renal impairment. These patients represented a high-risk group with an approximately 50% increased relative mortality risk when compared with patients who had normal renal function.12 Similar findings were observed in the Acute Decompensated Heart Failure National Registry (ADHERE) (see Chapter 25). In the Second Prospective Randomized study of Ibopamine on Mortality and Efficacy, impaired renal function was a stronger predictor of mortality than impaired LV function and NYHA functional class in patients with advanced HF (Fig. 25-5). Thus renal insufficiency is a strong, independent predictor of adverse outcomes in patients with HF. HF should be viewed as a continuum comprising four interrelated stages, as depicted in Figure 25-6.13 Stage A includes patients who are at high risk for developing HF, but without structural heart disease or symptoms of HF (e.g., patients with diabetes or hypertension). Stage B includes patients who have structural heart disease but without symptoms of HF (e.g., patients with a previous MI and asymptomatic LV dysfunction). Stage C includes patients with structural heart disease who have developed symptoms of HF (e.g., patients with a previous MI with shortness of breath and fatigue). Stage D includes patients with treatment-refractory HF requiring special interventions (e.g., patients with refractory HF who are awaiting cardiac transplantation). A simplified algorithm for the approach to the patient with HF is presented in Figure 25-7. The clinical assessment of patients with HFrEF is discussed in detail in Chapter 23, and the diagnosis and management of patients with HFpEF are discussed in detail in Chapter 27.
Management of Patients with Heart Failure with Reduced Ejection Fraction
Epidemiology
Etiology

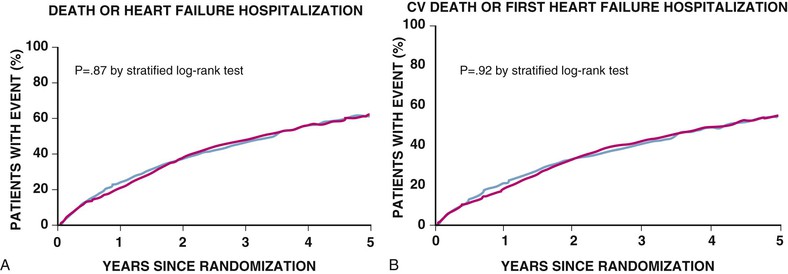
Prognosis
Biomarkers and Prognosis
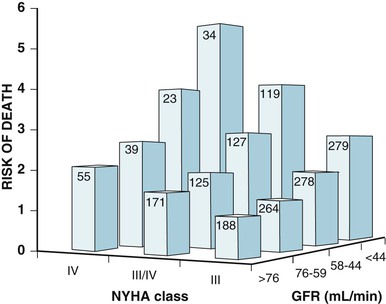
Renal Insufficiency
Approach to the Patient
Management of Patients with Heart Failure with Reduced Ejection Fraction
25