Fig. 64.1
Viewing the intrapleural space during medical thoracoscopy
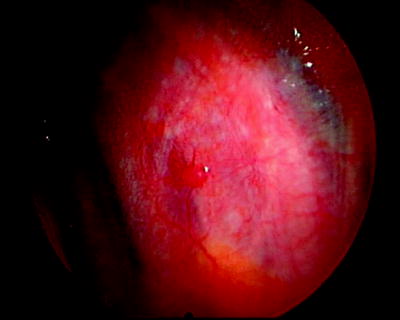
Fig. 64.2
Solitary parietal pleural tumor studding in a patient with an exudative pleural effusion of unknown etiology
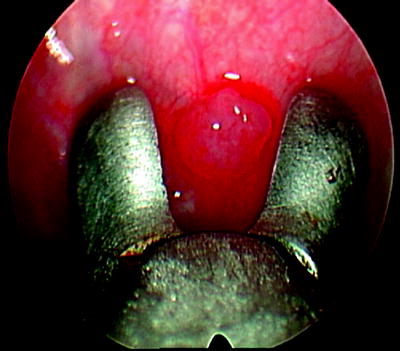
Fig. 64.3
Obtaining a biopsy of the single parietal pleural lesion under direct vision, using medical thoracoscopy. The pathology revealed adenocarcinoma from a beast primary
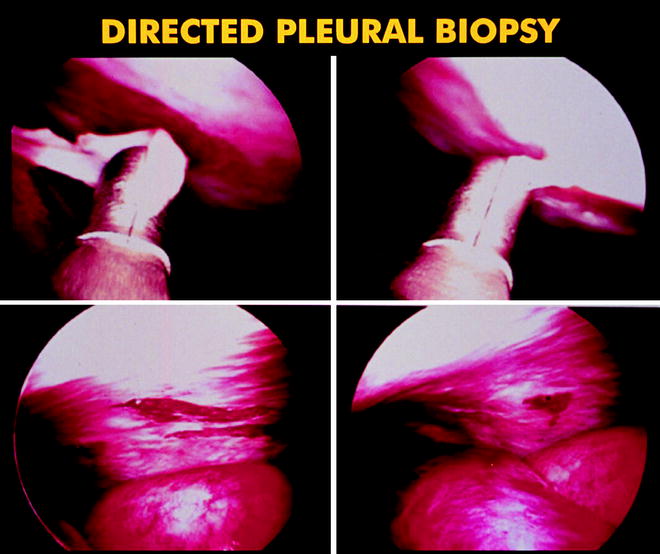
Fig. 64.4
Photo showing the sizable samples that can be obtained from the parietal pleura using medical thoracoscopy
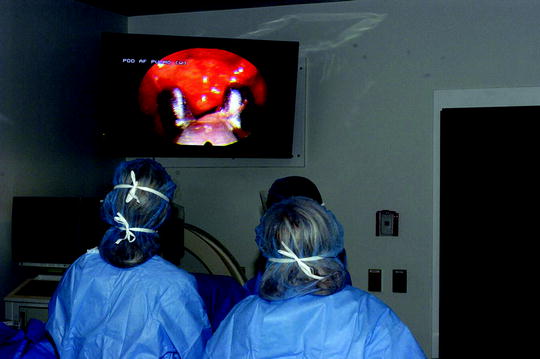
Fig. 64.5
Obtaining a parietal pleural biopsy during medical thoracoscopy
In the past, pathologists found it difficult to make a definitive diagnosis of malignant mesothelioma without large samples obtained during open thoracotomy or autopsy. With the availability of immunohistochemistry techniques, pathologists are now better able to make the diagnosis. By permitting direct visualization of lesions, pleuroscopy facilitates the choice of biopsy sites and allows accurate assessment of the degree of involvement of the diaphragmatic, parietal, visceral, and mediastinal pleura (Fig. 64.6). Boutin reported a sensitivity of thoracoscopic biopsy of 98% for the diagnosis of malignant mesothelioma, compared with 28% for pleural fluid cytology, 24% for closed-needle pleural biopsy, and 100% for surgical biopsy.
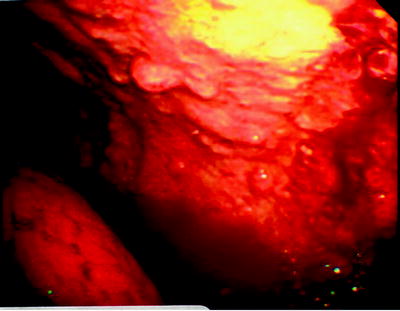

Fig. 64.6
Tumor studding along the parietal pleura. The biopsy revealed malignant mesothelioma
Bronchoscopy
The role of bronchoscopy in the diagnosis of malignant pleural effusions is limited and is not considered a routine part of the evaluation for a pleural effusion because of its low yield. A retrospective review, however, concluded that it may be useful in diagnosing bronchogenic carcinoma in patients with sizable cytology-negative pleural effusions who have hemoptysis, a lung mass, or atelectasis.
Treatment Options
While the interventional pulmonologist can play an important role in the accurate diagnosis of MPEs, an equally important, if not more frequent, role is in the treatment of MPEs. Since the presence of a MPE typically reflects advanced disease, the treatment options are generally palliative, not curative, so as to help relieve dyspnea, cough, and discomfort, thereby improving patients’ quality of life. The cause of dyspnea, the most frequent and often the most distressing symptom, is likely multifactorial and can be due to compressive atelectasis, decreased lung compliance causing increased work of breathing, and worsened ventilation-perfusion mismatching causing hypoxemia. These problems are potentially improved by the drainage of the MPE.
Observation
Given that the treatment of a MPE is almost always palliative, directed at relieving symptoms, it is appropriate for patients without symptoms to be simply observed or treated for the underlying malignancy by an oncologist without drainage of the effusion. While many types of MPEs will not respond to chemotherapy or radiation therapy, some tumor types may respond, such as small cell bronchogenic carcinoma, lymphoma, breast adenocarcinoma, and prostate adenocarcinoma. Treatments involving drainage of the effusion, as described below, can be initiated if the MPE increases in size and begins to cause symptoms.
Therapeutic Thoracentesis
All patients with a MPE will have likely undergone a thoracentesis as part of the diagnostic evaluation. In addition to playing an integral role in the diagnosis of a MPE, thoracentesis also plays a central role in the palliative treatment when a large volume of pleural fluid is drained. Indeed, if a MPE is strongly suspected in a patient with a newly noted pleural effusion, both a diagnostic and therapeutic thoracentesis can be combined in one procedure. By draining a large volume of fluid, one can assess whether the patient’s symptoms, particularly dyspnea, improve and whether the underlying lung reexpands radiographically. If symptoms significantly improve, other drainage procedures can be planned such as tube drainage and pleurodesis. If symptoms do not improve, other causes of the dyspnea should be considered, including underlying lung or heart disease, venous thromboembolism, chemotherapy- or radiation therapy-induced lung injury, tumor emboli, or pulmonary lymphangitic tumor spread. If the lung does not reexpand to touch the chest wall, one must consider pleural adhesions or lung entrapment by tumor preventing full expansion or atelectasis due to endobronchial obstruction by tumor. Such findings can affect diagnostic and treatment decisions; for example, diagnostic and therapeutic bronchoscopy might be indicated for endobronchial tumor obstruction, or pleurodesis might not be attempted if the visceral and parietal pleural surfaces cannot be apposed because of trapped lung.
The use of ultrasound guidance for localization of the pleural effusion and to determine the optimal entry point for drainage before performing thoracentesis is becoming standard practice. Besides localizing pleural fluid, it also helps identify adhesions and fluid loculations and helps avoid puncturing visceral organs. Pleural manometry, although not yet widely adopted, can be helpful in identifying patients with trapped lung because of abnormally negative intrapleural pressures and may also help prevent reexpansion pulmonary edema by stopping drainage when intrapleural pressures exceed −20 cm H2O (or when patients experience chest discomfort, which can be a surrogate for extremely negative intrapleural pressures.) The American Thoracic Society (ATS) and the most recent British Thoracic Society (BTS) practice guidelines continue to recommend limiting fluid withdrawal to <1.5 L during thoracentesis to avoid reexpansion pulmonary edema, in the absence of pleural manometry.
Large-volume thoracentesis is an important initial therapeutic measure for MPEs, but serial thoracentesis as the primary treatment modality is rarely a good option because of the propensity for the effusion to recur, the increased risk of multiple drainage procedures, and the increasing difficulty to completely drain the effusion because of adhesion formation and loculations with subsequent thoracenteses. In most cases, other procedures described below are generally better options because prevention of fluid reaccumulation is one of the goals of these procedures. Serial therapeutic thoracentesis should be reserved for patients with limited survival expectancy (<1 month) and poor performance status who cannot tolerate other drainage procedures.
Chest Tube Drainage and Pleurodesis
Adequate drainage of the MPE and prevention of fluid recurrence remains the main goal of the major treatment options. Prevention of fluid recurrence is most often achieved with chemical pleurodesis, which involves the instillation of a sclerosing agent into the pleural space. Several sclerosing agents have been used and described, all of which are meant to cause inflammation with fibrin deposition and consequent adhesion between the visceral and parietal pleural surfaces, thus preventing pleural fluid reaccumulation. Successful chemical pleurodesis requires the apposition of the pleural surfaces and thus reexpansion (at least partial, if not full) of the lung after pleural fluid drainage.
The two most common methods for draining the pleural fluid prior to instillation of a chemical sclerosant are chest tube drainage and thoracoscopic drainage. It had been previously assumed that large-bore chest tubes (at least a 24 F) were necessary for adequate drainage of the pleural space prior to chemical pleurodesis, but it has been shown in prospective, randomized control trials that smaller-bore chest tubes (10–14 F) provide adequate drainage with less discomfort compared to large-bore chest tubes. For chest tube drainage, the current BTS guidelines for the management of malignant pleural effusions recommend the insertion of a small-bore intercostal tube, controlled evacuation of fluid (initial drainage of 1.5 L, and 1.5 L at a time every 2 h) to prevent reexpansion pulmonary edema and radiographic confirmation of chest tube placement and lung reexpansion.
Many chemical sclerosants to achieve pleurodesis have been reported, but the most commonly used sclerosants are sterile talc, doxycycline, and bleomycin, with talc being the most commonly used. All three sclerosants can be instilled into the pleural space through a chest tube, although talc can also be instilled as a dry powder (poudrage) during medical thoracoscopy; this method will be described in the next section. The sclerosant of choice can be instilled as soon as the pleural effusion has been drained and when there is radiographic evidence of lung expansion; instillation does not need to be delayed until there is a predetermined amount of daily fluid drainage. Because the instillation of sclerosants (particularly doxycycline) is often painful to patients, lidocaine (3 mg/kg, maximum 250 mg) should be administered intrapleurally just prior to sclerosant administration, and premedication to alleviate pain and anxiety should be considered.
There have been numerous reports describing the efficacy of various sclerosing agents for pleurodesis, but there have been few high-quality comparative effectiveness trials to determine the best sclerosing agent. Meta-analyses have consistently identified talc as having the highest efficacy (whether given as a slurry through a chest tube or by poudrage during thoracoscopy) with success rates ranging from 70% to 100%. The typical dose of talc when given as a slurry is 5.0 g diluted in 50–100 ml of normal saline. Common adverse effects of talc, likely secondary to pleural inflammation, include pain and discomfort, fever (which is common and often transient), hypoxemia (in up to 30% of patients), and dyspnea. Less common side effects include pneumonia, arrhythmias, and empyema. Acute lung injury and acute respiratory distress syndrome (ARDS) due to talc has been described by several authors and does not appear to be related to dose or method of delivery but may be related to talc particle size. The talc available in Europe (mean particle size >20 μm) has not been reported to cause ARDS, as opposed to the talc available in the United States (the only FDA-approved preparation is Sclerosol®), which contains particles <10 μm. It has been hypothesized that talc with smaller particle sizes (<10 μm) can be systemically absorbed into the vascular beds and cause an inflammatory reaction in the lung resulting in an acute pneumonitis or ARDS. The incidence of respiratory failure in patients with MPE receiving talc slurry for pleurodesis was 4% in a prospective randomized trial. Despite the potential adverse effects, talc remains the most commonly used pleural sclerosant because of its wide availability, low expense, and good efficacy.
Tetracycline had been frequently used as a chemical sclerosant until its production ceased in the early 1990s. Doxycycline has been used as a substitute for tetracycline with successful pleurodesis rates of 60–81%. Common side effects include fever and pain, which can be severe and makes the use of intrapleural lidocaine and analgesics all the more important when using doxycycline. A typical doxycycline dose is 500 mg diluted in 50–100 ml of sterile normal saline instilled through the chest tube.
Bleomycin is an effective sclerosant with successful pleurodesis rates reported at 58–85%. Common side effects include fever, chest pain, and cough. It is not used commonly in the United States because of its relatively high cost, especially compared to talc, and its lack of demonstrated superiority over talc.
It is common practice to rotate the patient to different positions after instillation of the sclerosant to ensure intrapleural dispersion, but prospective studies have shown no advantage of rotational maneuvers over simply clamping the tube for 2 h. It has also been customary to remove the chest tube when daily pleural fluid drainage has fallen below a threshold level, such as 100–150 ml per day, but it is possible to remove it sooner as long as the chest x-ray shows adequate drainage for the effusion without diminishing effectiveness.
Thoracoscopic Drainage and Pleurodesis
Medical thoracoscopy can be used to achieve pleurodesis by completely draining the pleural space and instilling the sclerosing agent under direct vision, usually dry talc delivered as a poudrage (see Figs. 64.7 and 64.8). There are several theoretical advantages to thoracoscopic talc insufflation compared with talc slurry sclerosis. Medical thoracoscopy allows complete effusion drainage under direct visualization and optimal chest tube positioning. Talc is insufflated in a manner that allows even distribution over the entire visceral and parietal pleural surfaces. In contrast, the slurry of water-insoluble talc may gravitate to the dependent part of the pleural space shortly after instillation. Finally, in patients with an underlying malignancy but negative fluid cytology, parietal pleural biopsies of suspicious areas can be taken at the time of medical thoracoscopy, before proceeding with pleurodesis. A systematic review comparing the various treatment options to achieve pleurodesis in patients with MPE was published in the Cochrane Database in 2004. The comparison of thoracoscopic talc pleurodesis (TTP) and talc slurry pleurodesis favored thoracoscopic pleurodesis, with a relative risk for nonrecurrence of the effusion of 1.19 (95% CI, 1.04–1.36).
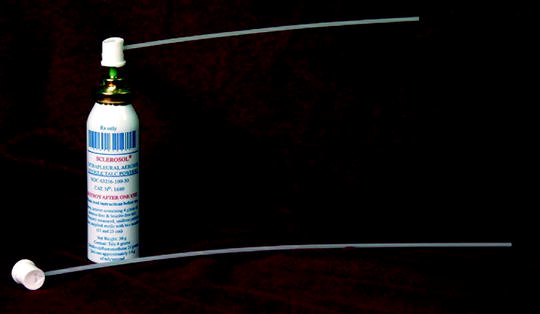
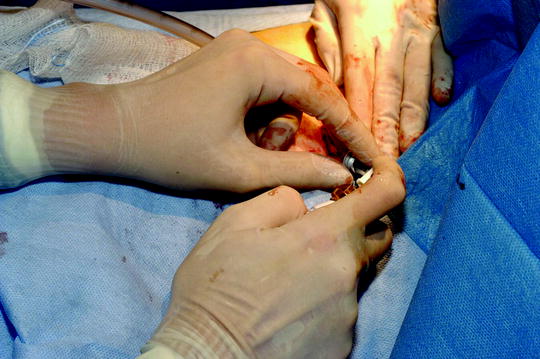

Fig. 64.7
Sterile talc that has been sterilized and packaged in a pressurized canister (Sclerosol®) for talc poudrage during medical thoracoscopy

Fig. 64.8
Performing talc poudrage using Sclerosol® during medical thoracoscopy
A large, multicenter randomized trial comparing talc poudrage with talc slurry was conducted by the North American Cooperative Oncology Groups in which a total of 482 patients were randomized to thoracoscopy with talc insufflation (n = 242) or tube thoracostomy with talc slurry (n = 240). Overall, no difference was detected in the percentage of patients with successful pleurodesis at 30 days (78% for TTP and 71% for talc slurry). However, in the subgroup of patients with primary lung or breast cancer, the success rate of TTP was found to be significantly higher than with talc slurry (65% vs. 50%, p = 0.014). Lung cancer and breast cancer are the first and second most common neoplasms causing malignant effusions, and these findings suggest that TTP may be a better option for a large proportion of patients with MPE. Moreover, a subgroup analysis of those patients with lung cancer or breast cancer who achieved lung expansion after drainage of the fluid (and therefore did not have radiographic evidence for trapped lung) and were alive at 30 days showed that TTP achieved successful pleurodesis more frequently compared to talc slurry (82% vs. 67%, p = 0.022).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


