TABLE 8.1 FEATURES OF CRITICAL LIMB ISCHEMIA | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
≥20/mm2, reactive hyperemia in capillary microscopy and laser Doppler, TcPO2 > 30 mm Hg) achieved a 1-year limb survival of 88%.
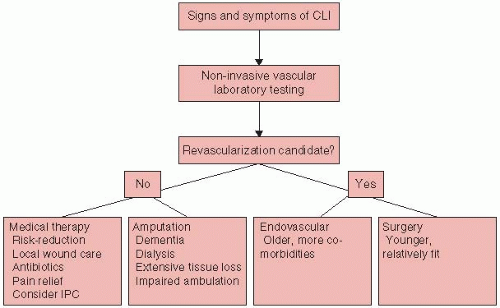
Atherosclerosis risk reduction. Atherosclerosis care is of great importance in the patient with CLI owing to these patients’ high risk for cardiovascular events. All patients with CLI should be considered to be candidates for antiplatelet agents and statins unless contraindicated.
Antiplatelet drugs: There is a case for treatment of all patients with CLI with long-term antiplatelet therapy, such as with aspirin or clopidogrel, based on the pathophysiologic importance of the platelet in thrombus propagation, which undoubtedly is a component in CLI. Recent small randomized trials in PAD and a more recent metaanalysis of previous prospective randomized trials that included PAD patients as a subset have questioned the efficacy of aspirin in PAD in reducing overall mortality or recurrent cardiovascular events except for stroke (Table 6.4). However, none of these studies specifically address the CLI population. In the absence of such data and in light of the critical importance of ongoing thrombosis in many patients with CLI, antiplatelet therapy with ASA (81 to 325 mg daily) alone or clopidogrel 75 mg daily is recommended. At this time, there are no data supporting one agent over the other or a combination except in post hoc analysis from CHARISMA in non-CLI patients with PAD. A small double-blind randomized controlled trial (n = 108) from the United Kingdom in CLI patients undergoing infrainguinal revascularization demonstrated that a preoperative regimen of clopidogrel (600 mg prior to surgery, and 75 mg daily for 3 days; n = 50) with ASA 75 mg reduced platelet activation and troponin release both before and after surgery.
Statins: Post hoc data from statin trials suggest a reduction in the need for amputations in subjects with a diagnosis of PAD. In the PREVENT-III trial of 1,404 patients with CLI, the use of statins was associated with reduced 1-year mortality rate. The NCEP-ATP III
guidelines support PAD as a risk equivalent with LDL and non-HDL goals similar to those with coronary heart disease. It is prudent to lower LDL to less than 70 mg/dL and non-HDL cholesterol as a secondary goal to less than 100 mg/dL in these patients.
Anticoagulation: The role of systemic anticoagulation in the setting of CLI remains controversial. Small trials have demonstrated benefits mainly in the subset of CLI patients undergoing peripheral bypass grafting. Based on post hoc analysis of the Dutch Bypass Oral Anticoagulants or Aspirin trial, patients who have undergone infrainguinal bypass graft surgery using autologous vein may benefit from oral anticoagulation with warfarin. The main trial did not show an advantage of oral anticoagulation, but the subset of patients receiving venous bypass grafts benefited from anticoagulation and a target international normalized ratio of 3.0 to 4.0; aspirin appeared better for prosthetic grafts. However, patients treated with oral anticoagulants had nearly twice the rate of major bleeding than patients taking aspirin. Anticoagulation for 3 months following bypass grafting is generally accepted to prevent acute graft thrombosis and thromboembolic complications in the acute phase. However, the decision to continue treatment long-term should be made on an individual basis. In general, anticoagulation for preserving graft patency may be considered in specific high-risk situations such as bypasses with comorbidities that increase the risk for thromboembolization, such as atrial fibrillation or low ejection fraction. There is some evidence that low-molecular-weight heparin administered for 3 months at 2,500 IU is better than aspirin and dipyridamole in maintaining femoropopliteal graft patency in patients with CLI undergoing salvage surgery. The CASPAR trial evaluated the efficacy of clopidogrel 75 mg + ASA 325 mg in comparison with ASA alone following belowknee bypass grafts. The primary composite outcome (death/indexgraft occlusion/revascularization/above-ankle amputation) occurred in an equal number of patients in both groups (Table 6.4). In a prespecified subgroup analysis, patients who underwent a prosthetic bypass graft demonstrated a benefit with the combination of ASA and clopidogrel without an increase in bleeding.
Pain control. Treatment of pain in the CLI patient is an important aspect of care. Pain should be assessed at every clinic visit and documented (a simple pain scale between 1 and 10 is useful). Although ultimate pain relief comes with improvement in blood flow with revascularization or amputation (if indicated), pain control is essential when these therapies are being planned. Narcotics are often required for complete pain relief.
Treatment of infection. Systemic antibiotics may be indicated for superadded infection or cellulitis. Infection may be suspected with the onset of rubor and tenderness in a wound. The use of antibiotics should
not delay more definitive treatment, which often requires a combination of prolonged antibiotic administration and surgical debridement of the infected bone. Osteomyelitis should be suspected if the ulcer area is greater than 2×2 cm, a probe can pass through tissue to bone, or the erythrocyte sedimentation rate is greater than 70 mm/h. Plain radiographs should be employed as the first imaging modality. Computed tomography may detect the presence of sequestrum, foreign body, or gas formation. Nuclear studies and magnetic resonance imaging offer the most sensitive and specific means of detecting osteomyelitis. Infections are often polymicrobial, especially in diabetics. Staphylococcus aureus is the most common pathogen cultured from bone samples, followed by S. epidermidis. Common Gram-negative pathogens include Escherichia coli, Klebsiella pneumoniae, Proteus sp., and Pseudomonas aeruginosa.
Wound care. The basic tenets of wound healing include assurance of adequate perfusion to the ischemic limb, adequate nutrition, and eradication of infection and mechanical features that inhibit healing. Table 8.2 presents guidelines for ischemic ulcer care. In general, adherence to these principles alone obviates the need for more expensive topical therapies. Debridement of infected wounds may be achieved by surgery, biosurgery (i.e., myiasis), hydrotherapy, negative pressure therapy, and wound dressings. Negative pressure wound therapy (Vacuum Assisted Closure, Kinetic Concepts Inc., San Antonio, TX) is a technique that uses subatmospheric pressure to remove excess fluid from the wound, which leads to improved oxygenation and blood flow. The technique is contraindicated in patients with thin, friable skin and in those with wounds secondary to neoplasm.
Footwear. Each CLI patient should be evaluated by a podiatrist to ensure that footwear is appropriate and not causing repetitive foot trauma.
Specific pharmacotherapy in CLI. Currently, no pharmacologic agent is approved by the Food and Drug Administration for the treatment of CLI. Prior trials using a number of drugs have been disappointing in their ability to reduce limb loss and overall morbidity and mortality associated with CLI.
Prostanoids and vasodilator therapy: Nine double-blind studies have demonstrated significant reductions in pain and ulcer size and three
studies have shown a reduced need for amputation with parenterally administered vasodilator prostaglandins such as prostaglandin E1. In general, the responses tend to be greater when these drugs are administered for 4 weeks rather than for shorter periods. Iloprost, a stable analogue of prostacyclin, is the most extensively investigated prostanoid. In one trial, patients who received iloprost were less likely to undergo a major amputation compared with patients in the placebo group (23 vs. 39%; P < 0.05) during treatment and followup, supporting its usage in patients with CLI, in patients who are unsuitable for a revascularization procedure, or in patients in whom such procedures have failed. These findings have not been replicated by more recent studies using oral iloprost or parenteral lipo-ecraprost as destination therapy or as adjunctive therapy immediately following distal revascularization. Based on available data, prostaglandins cannot be recommended as therapy for patients with CLI.
Evidence from randomized controlled studies to support the use of vasoactive drugs, such as cilostazol, in CLI is lacking.
TABLE 8.2 GENERAL PRINCIPLES OF ISCHEMIC ULCER CARE
Keep area assiduously clean
Saline dressings three or four times a day when the ulcer is “weeping”
Transition to dry dressings once ulcer is dry
Avoid excessive debridement and topical antibiotics
Angiogenic growth factors and stem cell therapy: Single growth factor approaches including those delivered via plasmid or adenoviral approaches including VEGF-A and Hepatocyte Growth Factor were evaluated as part of early Phase I/IIa trials but are not being pursued owing to lack of convincing effect. A recent large trial in 525 patients with CLI comparing FGF-1 delivered as a plasmid versus placebo showed that FGF-1 was not effective in reducing amputations or deaths in patients with CLI. Early phase I data involving delivery of transcription factors such as hypoxia inducible growth factor (HIF-1α) appear promising. These need to be rigorously tested in randomized controlled clinical trials. A large number of open-label Phase I trials with bone marrow mononuclear cells have demonstrated benefit in small numbers of patients. The benefit of these approaches needs to be tested in larger Phase II/III trials.
Mechanical therapies: Intermittent pneumatic compression (IPC) may provide symptom relief and wound healing for CLI patients who are not candidates for vascular reconstruction. A retrospective study of CLI patients with nonhealing wounds in whom all means of additional revascularization had been exhausted found that the below-knee amputation (BKA) rate for IPC patients was 42 versus 83% for controls at 18-months follow-up. IPC requires an intensive time commitment; patients in the active treatment group received 6 hours of IPC (ArterialFlow, DJO, Vista, CA) each day in addition to standard wound care.
Spinal cord stimulation (SCS) and sympathectomy: The use of SCS remains controversial. A Cochrane review concluded that SCS was superior to medical management for treating CLI patients with unreconstructable vascular disease. However, a meta-analysis of five
randomized trials showed that SCS was no better than medical therapy alone in preventing amputations; at least 14 patients must be treated to avoid one amputation at a cost of more than $150,000 per limb saved. Another option for selected patients is surgical or chemical lumbar sympathectomy, which improves skin blood flow in the leg and foot and is associated with 1-year limb-salvage rates of 58 to 61%. Because of high cost and uncertain benefit, use of hyperbaric oxygen may be limited to reducing the risk of major amputation in patients with diabetic foot ulcers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree