(1)
University of Ottawa The Ottawa Hospital, Ottawa, ON, Canada
An appropriate classification of cardiomyopathies is as follows:
1.
Hypertrophic cardiomyopathy
2.
Dilated cardiomyopathy
3.
Restrictive cardiomyopathy
4.
Arrhythmogenic right ventricular cardiomyopathy (right ventricular dysplasia)
5.
Unclassified cardiomyopathy: diseases that do not have features of 1 through 4 and include fibroelastosis and mitochondrial disease
6.
Specific cardiomyopathies (specific heart muscle diseases formerly called secondary cardiomyopathy)
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is now recognized as a genetic disorder caused by more than 1,400 mutations in 11 or more genes encoding proteins of the cardiac sarcomere (Maron and Maron 2013). HCM is often inherited in an autosomal dominant pattern, but there are many patients without any relatives who are known to have the disease (Fifer and Vlahakes 2008).
HCM is the most common genetic cardiovascular disease. In the majority of patients the disease runs a benign course, but is the most common cause of sudden cardiac death in young individuals including athletes. Maron et al. (1986) indicate that HCM may cause sudden death in individuals of all ages. But sudden death occurs in asymptomatic or mildly symptomatic patients (Maron 2010).
Sudden death results primarily from ventricular arrhythmias that, in turn, are likely related to an abnormal substrate of disorganized muscle cell structure and a multitude of possible inciting events, such as ischemia, abnormal autonomic milieu, atrial arrhythmias, or bradycardia. These devastating events occur infrequently, but pose a management dilemma for clinicians (Nishimura and Ommen 2007). Approximately 70 % of patients have outflow obstruction at rest or with exercise; about 33 % will have the non-obstructive form without capacity to generate significant outflow gradients (Maron et al. 2006).
“In children with hypertrophic cardiomyopathy, the risk of death or heart transplantation was greatest for those who presented as infants or with inborn errors of metabolism or with mixed hypertrophic and dilated or restrictive cardiomyopathy” (Lipshultz et al. 2013).
Pathophysiology
Most patients show asymmetric hypertrophy of the septum and a hypertrophied non-dilated left and or right ventricle. The septum may be diffusely hypertrophied or only in its upper, mid, or apical portion. Hypertrophy extends to the free wall of the left ventricle. There is decreased compliance and incomplete relaxation of the thickened and stiff left ventricular muscle that causes impedance to filling of the ventricles during diastole (diastolic dysfunction). The rapid powerful contraction of hypertrophied left ventricle expels most of its contents during the first half of systole. This hyperdynamic systolic function is apparent in most patients with HCM.
The anterior leaflet of the mitral valve is displaced toward the hypertrophied septum. Mitral regurgitation is virtually always present in the obstructive phase of the disease. The sequence of events is eject, obstruct, and leak. A variable LV outflow pressure gradient at rest occurs in approximately 35 % of patients. A further 25 % develop a similar gradient precipitated by conditions that increase myocardial contractility or decrease ventricular volume. Thus, diuretics and other causes of hypovolemia and preload-reducing agents that reduce the volume of the small ventricular cavity may worsen outflow tract obstruction.
Clinical Diagnosis
The palpable left atrial beat preceding the LV thrust is a most important sign because it can occur in the absence of gradient or murmur.
A murmur is heard and has typical characteristics, but the entire examination may reveal little or no abnormalities depending on the stage of the disease.
The murmur has typical features:
Crescendo–decrescendo starts well after the first heart sound (S1) and ends well before the second heart sound (S2). It is best heard between the apex and left sternal border.
Radiates poorly to the neck, if at all.
Intensity increases with maneuvers or drugs that decrease preload (Valsalva, standing, amyl nitrite) and decreases in intensity with an increase in afterload (squatting, hand grip, phenylephrine).
Easy to distinguish from aortic valvular stenosis, in which the murmur starts soon after the S1 and radiates well to the neck.
A mitral regurgitant murmur is often heard in the last half of systole with radiation to the axilla. It is usually associated with an outflow tract gradient.
Diagnosis of HCM requires the finding of a hypertrophied non-dilated left ventricle without evidence of any other cardiac or systemic disease (e.g., systemic hypertension) that could produce the extent of hypertrophy observed (Maron et al. 2003).
Electrocardiographic Findings
The ECG is abnormal in >90 % of patients with significant symptomatic HCM and about ~85 % abnormal in asymptomatic patients.
McLeod et al. (2009) in a study concluded: almost 6 % of patients presenting with demonstrable echocardiographic evidence of HCM had a normal ECG at the time of diagnosis. This subset of patients with normal ECG-HCM appears to exhibit a less severe phenotype with better cardiovascular outcomes. None of these patients had a cardiac death at follow-up.
The ECG may be abnormal when the echocardiogram shows no significant abnormality.
ECG findings include:
Deep narrow Q waves in about 30 % of subjects in leads II, III, aVF, V5, and V6, or in I, aVL, V5, and V6, and rarely V1 through V3, which at times reflect septal hypertrophy and may mimic myocardial infarction
Intraventricular conduction delay in over 80 %.
High QRS voltage LV hypertrophy (LVH).
Left atrial abnormality indicating enlargement and/or hypertrophy.
Diffuse T-wave changes in some patients or T waves of LVH.
Giant inverted T waves, very high precordial QRS voltage with apical HCM
There can be marked ST-segment elevation in V2–V4 with prominent negative waves in V2–V4 as shown in Fig. 23-1.
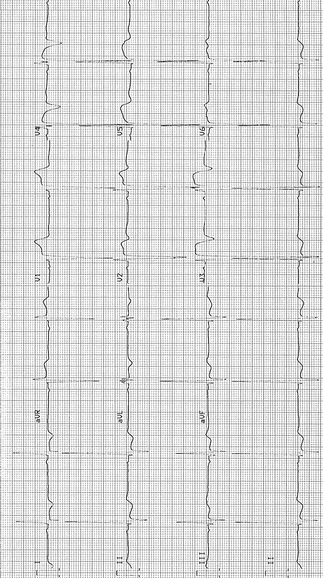
Fig. 23-1.
Hypertrophic cardiomyopathy. ECG shows voltage criteria for LVH. There is marked ST-segment elevation V2–V4 with prominent negative waves in V2–V4; Q waves in leads II–III, aVF, V5, and V6 in keeping with septal hypertrophy. From an asymptomatic 20 year old, played soccer, rugby, and hockey from age 15–20. Routine physical revealed a grade II systolic murmur. Echocardiogram showed marked asymmetric septal hypertrophy, septal thickness 36 mm (normal less than 11 mm). No dynamic outflow obstruction at rest. At age 22, one episode of presyncope and one episode of syncope after exercise; ICD implanted. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.
ST-segment depression in some.
PR interval occasionally short; preexcitation may be seen.
Over time atrial fibrillation occurs in ~20 %
The ECG is a good screening test because <7 % of individuals with HCM are expected to have a normal electrocardiogram, and these patients probably are at low risk. Sensitivity and specificity are equal to that of echocardiography.
Echocardiography
Two-dimensional echocardiographic observation of a LV myocardial segment of 1.5 cm or more in a normal-sized adult is considered diagnostic if there is no other evident cause. Continuous-wave Doppler echocardiography defines the degree of LV outflow-tract gradient. Asymmetric septal hypertrophy involving most of the septum is the most common variant form of hypertrophy; see Fig. 23-2.
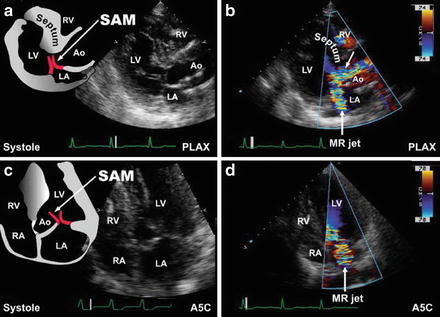

Fig. 23-2.
Dynamic left ventricular outflow tract obstruction. Dynamic left ventricular outflow tract obstruction with systolic anterior motion of the mitral valve leaflets (SAM) are shown in this parasternal long-axis (PLAX) view (a). Suboptimal mitral leaflet coaptation accompanies SAM and is typically accompanied by a posteriorly directed mitral regurgitant jet. Note the turbulence created within the left ventricular outflow tract (arrow, b). Apical five-chamber (A5C) views of the same features above are shown (c, d). Reproduced with permission from Bulwer BE, Solomon SD: Cardiomyopathies. In: Solomon SD, Bulwer BE, editors. Essential Echocardiography: A Practical Handbook. Totowa, NJ: Humana Press, 2007; p. 175. With kind permission from Springer Science + Business Media.
Therapy
Management necessitates the accomplishment of the following:
1.
Activity restriction with avoidance of volume depletion.
2.
Control of symptoms.
3.
Prevention of sudden death.
4.
Screening of relatives.
5.
Symptomatic relief (unrelated to protection from sudden death) can often be accomplished by pharmacologic therapy designed to: Block the effects of catecholamines and diminish myocardial contractility that exacerbate outflow tract obstruction. Slowing of the heart rate to 50–60 beats per minute (BPM) to enhance diastolic filling. Administration of an appropriate beta-blocking drug (timolol, carvedilol, or nebivolol, propranolol in nonsmokers) and in carefully selected cases, the calcium antagonist, verapamil.
Some patients with obstructive HCM may benefit from disopyramide, which shares a negative inotropic action with beta-blockers and verapamil, and because of its atrial antiarrhythmic properties, disopyramide may be of particular benefit in patients with atrial fibrillation.
Patients must be instructed to avoid strenuous competitive exercise because it can cause sudden death. A decrease in ventricular volume or increase in ventricular contractility increases the outflow gradient.
Dehydration and the use of preload-reducing agents, such as diuretics, nitrates, or angiotensin converting enzyme (ACE) inhibitors, should be avoided. Beta-agonists increase contractility and are contraindicated.
Digoxin increases contractility and its use should be avoided, except in the management of chronic atrial fibrillation, a fast ventricular response uncontrolled by amiodarone, beta-blockers, or verapamil.
An algorithm for the management of symptomatic HCM in the absence of high risk advises the following (Fifer and Vlahakes 2008):
Commence a beta-blocking drug (experience mainly with propranolol).
If there is a contraindication to a beta-blocker or adverse effects, verapamil can be tried. Caution: watch for bradycardia, sinus arrest, AV block, hypotension, and heart failure.
In the presence of significant LV outflow tract gradient and persistent symptoms, add disopyramide with caution or consider mechanical therapy.
Refractory symptoms despite above treatment consider mechanical therapy:
Septal myectomy or ablation—(Fifer and Vlahakes 2008)
Beta-Adrenergic Blocking Agents
A study with propranolol was done in 22 patients with HOCM. Average propranolol dosage was 462 mg/day. Mean follow-up was 5 years. Dyspnea, angina, palpitations, dizziness, and syncope all improved (by 58–100 %) on propranolol (Frank et al. 1978).
Beneficial effects of beta-blocking agents include the following:
Decrease in myocardial contractility causes a decrease in “venturi” effect and therefore less obstruction.
Relief of dyspnea in about 40 % of patients.
Significant relief of angina in 33–66 % of patients.
The heart rate should be maintained between 52 and 60 BPM; this results in an improvement in ventricular filling during prolonged diastole; also increased coronary filling occurs because of prolongation of the diastolic interval and relaxation of the ventricular muscle.
Improvement in diastolic dysfunction.
Partial control of supraventricular and ventricular arrhythmias.
The therapeutic activity of beta-blockers, particularly propranolol, metabolized in the liver, and calcium antagonists are blunted by cigarette smoking. It is important for patients with HCM to desist smoking because of other adverse effects, as well as the decrease in effectiveness of the two major pharmacologic interventions.
Only lipophilic beta-blocking agents have been shown to prevent sudden cardiac death (timolol in smokers and nonsmokers, propranolol in nonsmokers). These agents are advisable.
Atenolol is hydrophilic, poorly effective, and should not be used. Sufficient attention has not been paid by the medical profession and researchers, regarding the subtle differences that exist amongst the available beta-blocking drugs (Khan 2005).
Nebivolol reduces P-wave dispersion on the electrocardiogram, which might attenuate the risk of atrial fibrillation (Tuncer et al. 2008).
Propranolol: Supplied as 20-, 40-, 80-, and 120-mg tablets (Inderal LA): 80, 120, and 160 mg. A dosage of 10 mg three times daily is given and increased slowly to 120–240 mg daily. A slow buildup of the dosage to 320 mg may be required. Propranolol is not effective in smokers.
Calcium Antagonists
Considerable experience with verapamil is now available, but the initial high expectations have not materialized, and the drug has caused deaths. Verapamil decreases dyspnea and increases exercise capacity in some patients but does not improve survival and has precipitated life-threatening pulmonary edema in a significant number of patients; these vasodilators can unpredictably increase the obstruction with resultant pulmonary edema, cardiogenic shock, and death. It is contraindicated in patients with severe obstruction or end-stage disease associated with ventricular dilation and HF. Verapamil: a dosage of 40 mg three times daily or 80 mg twice daily is used and increases slowly over weeks to 240–360 mg daily under close observation.
Implantable Cardioverter-Defibrillators
Implantable cardioverter-defibrillators (ICDs) are an effective therapy to prevent sudden death in patients with HCM. The decision to recommend an ICD is by no means a simple task and raises concerns regarding overtreatment because many individuals run a benign course.
ICD-related complications include infection in about 5 %, lead fractures, and fatal device malfunction. Also, there is a risk of severe tricuspid regurgitation after device implantation. Patients with two or more risk factors likely present a high-enough risk to warrant implantation of an ICD (Nishimura and Ommen 2007).
Maron provided an extensive review relating insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy (Maron 2010).
“The risk-factor algorithm used in hypertrophic cardiomyopathy is incomplete, as shown by infrequent sudden deaths in patients judged clinically not to be at high risk. A subgroup of patients with genetic mutations but without left-ventricular hypertrophy has emerged, with unresolved natural history. Now, after more than 50 years, hypertrophic cardiomyopathy has been transformed from a rare and largely untreatable disorder to a common genetic disease with management strategies that permit realistic aspirations for restored quality of life and advanced longevity” (Maron and Maron 2013).< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


