Prevalence in study series (%)
Atrioventricular block
26–67
Bundle branch block
12–61
Atrial arrhythmias
23–25
Ventricular arrhythmias
11–73
Sudden cardiac death
12–65
Congestive heart failure
10–30
Conduction System Disease
In cardiac sarcoidosis, granulomatous infiltration of the basal interventricular septum with resultant inflammation and subsequent scarring can cause injury to the various elements of the cardiac conduction system. This can result in a variety of conduction disturbances leading to bundle branch block or any level of atrioventricular block. Because of the progressive nature of CS, the level and severity of conduction block may also progress (Fig. 9.1).
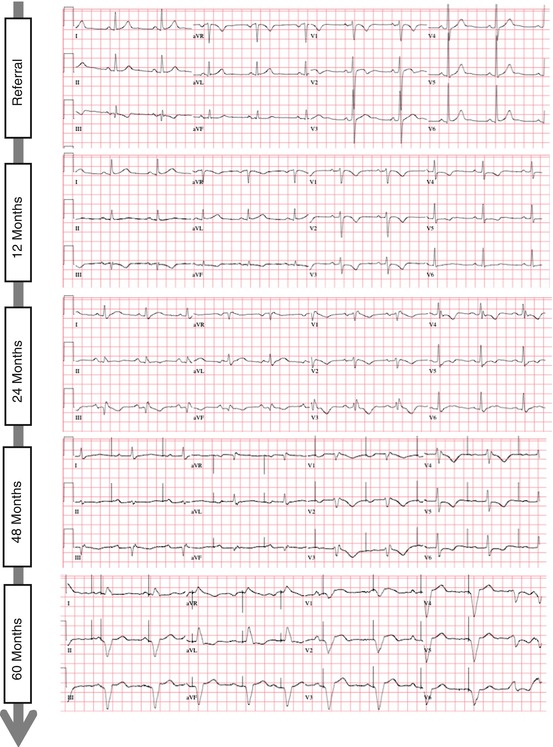

Fig. 9.1
Electrocardiographic progression of conduction system disease in a patient with cardiac sarcoidosis. Shown here is the progression (sequential ECGs, from top-to-bottom) in one patient over a period of 5 years from (1) sinus rhythm with a narrow QRS complex, to (2) first degree AV delay, to (3) RBBB, to (4) sinus node dysfunction with the need for atrial pacing, to (5) complete heart block with ventricular pacing
Bundle Branch Block
Bundle branch block has been observed on surface electrocardiograms in 12–61 % of cases of CS, depending on study series [1–4]. Both the original and 2006 revised Japanese Ministry of Health and Welfare guidelines for the diagnosis of CS use RBBB as one of the ECG abnormalities constituting a minor diagnostic criterion [5]. While neither sensitive nor specific, both RBBB and LBBB are seen more commonly in patients with CS than those with sarcoidosis without myocardial involvement [4], and should prompt further investigations. Further studies are also needed to determine whether the presence of bundle branch block has prognostic implications for the future development of complete atrioventricular block or ventricular arrhythmias and sudden death in these patients.
Other Asymptomatic Electrocardiographic Findings
With inflammation and subsequent scarring in CS, areas of delayed myocardial activation are expected, and this can manifest as fragmentation on the surface ECG. While not included in previously-published diagnostic guidelines, QRS complex fragmentation, defined by the presence of an additional R wave (R′), notching in the nadir of the S wave, or the presence of >1 R′ in two anatomically contiguous leads (Fig. 9.2), was shown in two study series to carry more diagnostic value than RBBB alone [4, 6].
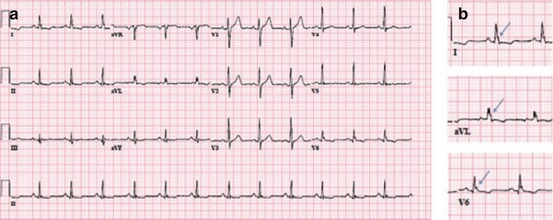

Fig. 9.2
ECG demonstrating QRS complex fragmentation and repolarization abnormalities (panel a), particularly notable in the lateral leads (arrows, panel b)
The signal-averaged ECG (SAECG) has utility in the detection of abnormal activation characterized by late potentials, and may also have both diagnostic and prognostic value [7]. Unlike the standard 12-lead ECG recording, requiring only a few seconds, SAECG recording requires up to 10 min, as multiple QRS potentials are averaged to allow both for the removal of interference due to skeletal muscle and for the detection of low amplitude, high frequency late potentials. While originally developed as a means of risk stratification in patients with coronary disease and cardiomyopathies to detect substrate for reentry, further investigations are needed to assess the abnormal SAECG as a risk marker for ventricular arrhythmias in CS.
Atrioventricular Block
Conduction block can range from first-degree atrioventricular delay to complete atrioventricular block, and the severity of block can progress with progression of inflammation and scar. Complete atrioventricular block (CAVB) is the one of the most common findings in patients with clinically-evident cardiac sarcoidosis, with a prevalence of 25 % in one retrospective analysis [1]. While usually felt to be related to infiltration of the conduction system itself, granulomatous involvement of the AV nodal artery has also been described as a cause of atrioventricular block in sarcoidosis [3]. CAVB often occurs at a younger age in patients with sarcoidosis than in individuals with complete heart block due to other etiologies.
Treatment of patients with high grade atrioventricular block with permanent pacing should be performed in accordance with published guidelines [8]. However, the presence of AV block likely signifies extensive myocardial involvement from sarcoidosis granuloma and portends a higher risk of future ventricular arrhythmias [9]. For this reason, the recently-published expert consensus statement from the Heart Rhythm Society related to the management of CS gives a Class IIa recommendation for ICD implantation (regardless of left ventricular ejection fraction) for the primary prevention of sudden cardiac death in CS if the patient meets an indication for pacing [10]. And, while there are no specific data related to the use of cardiac resynchronization therapy in CS patients, relevant recommendations based on major trials investigating biventricular pacing from the general device guidelines should apply to CS patients [8].
CAVB can be reversible in CS (see case vignette on recovery of atrioventricular conduction in Chap. 14), and there may be a role for immunosuppression in attempt to reverse CAVB. Kato et al. described their experience with 20 patients with CAVB and preserved left ventricular function [11]. AVB resolved in four of seven patients treated with corticosteroids (57 %), but did not improve in any of the untreated patients. In another study, atrioventricular conduction improved to normal in four of eight CAVB patients with relatively preserved left ventricular function, but in none of the four patients with advanced left ventricular dysfunction when given steroid treatment [12]. Accordingly, current guidelines state (with a Class IIa recommendation) that immunosuppression can be useful in CS patients with Mobitz II or third degree heart block.
Lastly, it should be noted that CAVB in young patients (less than 60 years of age) should prompt evaluation for sarcoidosis, even in those who do not carry a previous diagnosis of extracardiac sarcoidosis. CAVB can be the initial manifestation of cardiac involvement in patients with a prior diagnosis of systemic sarcoidosis, as well as the first clinical manifestation of sarcoidosis from any organ. This was the case in a Japanese study of 89 consecutive patients with no known history of sarcoidosis with high-grade atrioventricular block requiring permanent dual chamber pacemaker implantation that were prospectively evaluated for cardiac sarcoidosis. Ten cases (11.2 %) of cardiac sarcoidosis were diagnosed, most frequently in young women aged 40–69 (32 %) [13]. Kandolin et al. investigated 72 patients (age <55 years) with unexplained AV block and found biopsy-verified CS in 14 (19 %) and ‘probable’ CS in 4 (6 %) of the 72 patients [14]. Patients with sarcoidosis had a significantly more adverse prognosis when compared to patients with idiopathic AV block in this series.
Sinus Node Dysfunction
Extensive granulatomous lesions in the sinoatrial node subendocardium have previously been described in autopsy series [15, 16], and sinus node dysfunction may be an under-recognized manifestation of CS [17]. Figure 9.3 shows an electrocardiogram of a patient with sinus node dysfunction and CS, ultimately leading to sinus arrest, and ultimately a polymorphic VT arrest. Patients with sinus node dysfunction may have an indication for permanent pacing, and if this is the case, dual chamber ICD implantation may be reasonable.
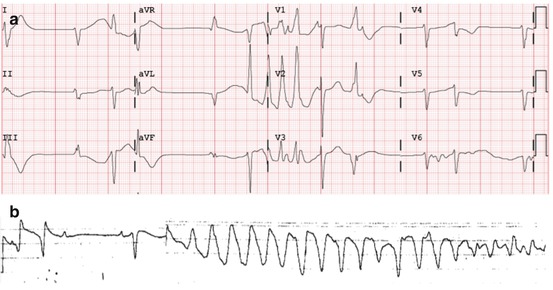

Fig. 9.3
A 12-lead ECG in a patient with CS showing sinus arrest, inferior QRS fragmentation, and QT prolongation with EADs (a), ultimately leading to a polymorphic VT arrest (b)
Atrial Arrhythmias
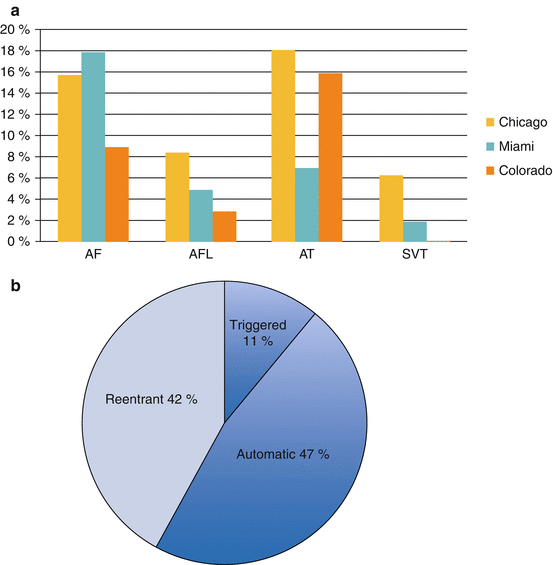
Supraventricular arrhythmias and atrial fibrillation are common in CS, with a prevalence ranging from 23 to 36 % (Fig. 9.4) [18–20]. In a study of 100 consecutive patients with biopsy-proven systemic sarcoidosis and evidence of cardiac involvement by MRI, PET, or endomyocardial biopsy, a 32 % prevalence of supraventricular arrhythmias was observed over a mean follow-up period of 5.8 years. Arrhythmias were documented by ECG, device interrogation data, or ambulatory telemetry monitoring. Atrial fibrillation was the most common supraventricular arrhythmia reported, accounting for 56 % of those described. After multivariate analysis, left-atrial enlargement was the only parameter significantly associated with supraventricular arrhythmias (HR 6.12, 2.2–17.1, P < 0.01) [18].
A similar prevalence of atrial arrhythmias was described in a separate retrospective series, in which 16/44 (36 %) patients with evidence of CS by cardiac magnetic resonance imaging (CMR) had documented atrial arrhythmias, the most common of which was atrial tachycardia (18 %). In the 26 patients with ICDs in this cohort, 11.5 % received inappropriate ICD therapies for atrial arrhythmias [20]. This was similar to the 12 % incidence of inappropriate therapies in a separate study [21].
Atrial arrhythmias arise from a variety of mechanisms in CS. In a third recently-investigated series, 15/65 patients (23 %) with CS experienced 28 distinct symptomatic supraventricular arrhythmias (9 Atrial Fibrillation; 3 Atrial Flutter; 16 Atrial Tachycardia). These patients ultimately underwent electrophysiologic testing to characterize the arrhythmias. The mechanism of atrial arrhythmias was determined to be from triggered activity in 11 %, abnormal automaticity in 47 %, and reentrant in 42 % of the non-AF atrial arrhythmias. Inflammation and edema associated with the initial infiltrative stage and scar that manifests as the disease progresses to the fibrotic stage likely independently account for the differing mechanisms observed.
The variety of mechanisms and differing substrate for atrial arrhythmias in CS suggests that management of atrial arrhythmias may require a multi-faceted approach including immunosuppression, antiarrhythmic therapy, catheter ablation, or a combination of these strategies for arrhythmia control. Immunosuppression is discussed in greater detail in subsequent chapters, though the three study series described above each employed immunosuppression with variable results. In one of the series, the overall burden of atrial fibrillation appeared to decrease with immunosuppression [19]. Anti-arrhythmic therapy might include the use of class IC agents, class III agents, or beta-blockers. The efficacy of these drugs for the control of atrial arrhythmias has not been specifically studied in CS.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree