Chapter 70 Malignant Pleural Mesothelioma
Pathogenesis
1. Mesothelioma cells have increased or dysregulated growth through both production of and response to various growth factors.
2. Mesothelioma cells express telomerase and therefore avoid telomere shortening (i.e., become “immortalized”).
3. Tumor suppressor genes such as p16 and p14, which are important in the Rb and p53 pathways, are absent in mesothelioma, allowing the tumor to avoid antigrowth signals.
4. Tumor cells evade apoptosis through increased activity of the antiapoptosis molecule Bcl-2.
5. Increased angiogenesis occurs through production of angiogenic factors such as vascular endothelial growth factor.
6. Malignant mesothelioma exists in a collagenous environment, and matrix interactions and regulation of this environment likely are related to tumor growth.
Pathology
Histology
Epithelial Subtype
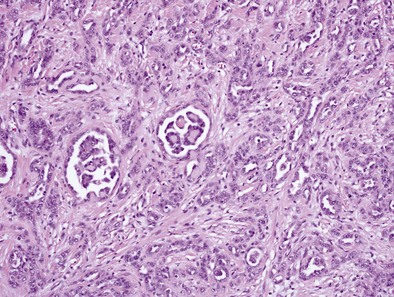
Epithelioid MPMs are the most common subtype and can be further characterized to a number of patterns. The tubulopapillary pattern contains a mixture of tubules and papillary structures (Figure 70-1). Adenomatoid and acinar patterns contain glandlike structures that should be differentiated from adenocarcinomas of other origin (e.g., lung). The solid, well-differentiated pattern has “nests” or sheets of cells that may resemble reactive mesothelium. Differentiating features from reactive mesothelial hyperplasia include stromal invasion, presence of necrosis and expansile nodules, as well as immunohistochemical staining patterns. Other types include solid, poorly differentiated, clear cell, and others.
Sarcomatous Subtype
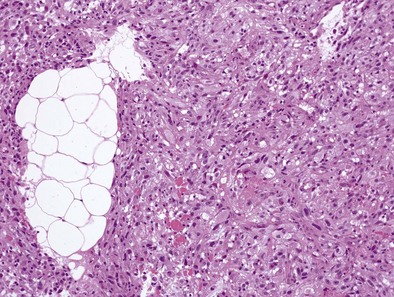
Sarcomatoid MPMs usually show spindle cells, often have more necrosis and atypical cells than epithelioid patterns, may contain anaplastic or giant cells, and include the lymphohistiocytoid and desmoplastic patterns (Figure 70-2). The desmoplastic pattern contains dense areas of collagen with small amounts of tumor cells, without significant atypia, and can be difficult to differentiate from nonmalignant pleural conditions such as fibrosing pleuritis; differentiating features include stromal invasion and disorganized growth with uneven thickness.
Immunohisto/Cytochemistry
• Reactive mesothelial hyperplasia and epithelioid MPM may be differentiated by a combination of morphologic features and IHC staining; desmin is often positive in reactive hyperplasia, and epithelial membrane antigen (EMA) and p53 may be positive in epithelial MPM.
• Malignant mesothelioma (MM) versus metastatic malignancy (often adenocarcinoma) is a common differential diagnosis. Adenocarcinomas often contain intracellular mucin on periodic acid–Schiff stain after digestion, which is only rarely seen in MM. As a general approach, initial IHC workup may include two mesothelial markers and two markers for the malignancy being considered, as well as pancytokeratin, which is positive in almost all MPMs, except less common sarcomatoid variants. If the tumor is keratin negative, panels for other cancers (e.g., melanoma, lymphoma) would be considered.
• Positive mesothelioma markers include calretinin, keratin 5/6, WT-1 protein, and podoplanin (D2-40). Markers that favor lung adenocarcinoma include Ber-EP4, which is positive in 95% to 100% of cases, compared to less than 20% of mesotheliomas. Carcinoembryonic antigen (CEA), TTF-1, MOC-31, BG8, B72.3, and CD15 are often positive in adenocarcinoma but only in up to 10% of mesotheliomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree