Magnetic Resonance of Cardiomyopathies and Myocarditis
Gobinath Nadeshalingam
Iacopo Carbone
Matthias G. Friedrich
Cardiomyopathies are chronic, progressive myocardial diseases with distinct morphologic, functional, and electrophysiologic characteristics and have been classified into four categories: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM), and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) (1).
While genetic defects are considered primarily responsible for HCM (2), other factors such as inflammatory or toxic injury or infiltration, with or without a genetic predisposition of the myocardium may contribute to the development of DCM (3), ARVC (4), and RCM.
In clinical settings, cardiomyopathies are typically diagnosed after exclusion of other cardiovascular causes, mainly explaining coronary artery disease. Importantly, the type of cardiomyopathy has a strong impact on therapy and prognosis. Therapy can be guided by the etiology, the disease stage, and severity. Thus, imaging techniques are of paramount importance for both diagnosis and therapy. The modalities frequently used in these diseases are echocardiography, conventional angiography, radionuclide ventriculography, and cardiovascular magnetic resonance (CVMR) imaging.
THE ROLE OF OTHER DIAGNOSTIC MODALITIES IN CARDIOMYOPATHIES
In clinical routine, transthoracic echocardiography serves as the standard imaging technique. It is easily accessible, noninvasive, and fast. But although there are a variety of applications of echocardiography in cardiomyopathies (5), results feature substantial interstudy and interobserver variability, which is a limiting factor in their use (6,7 and 8). Thus, the reliability of phenotyping a patient with a cardiomyopathy may be limited and follow-up data may be inconsistent, especially when different observers are involved (9,10). The use of transesophageal echocardiography (TEE) or techniques such as acoustic quantification (11), automated border detection (12), application of contrast (13), and three-dimensional (3D) postprocessing (14) may improve image quality and output, yet the endocardial border often remains difficult to detect, especially in the apex (15). Comparative studies indicate that 3D postprocessed echocardiography may be as accurate as CVMR (16,17). Still, the reliability of volumetric results relies on the quality of the raw image data set, which depending on the disease may be nondiagnostic in approximately 15% of patients. With respect to cardiomyopathies, one of the most important limitations of echocardiography (as well as other “ray” modalities) is the lack of techniques to analyze tissue pathology itself. Despite the initial hope to
identify specific pathology-related changes of echogenicity (18), results to date have generally been disappointing. Thus, echocardiography can be considered the modality of choice for the initial diagnostic workup, but has limited value for a more detailed disease myocardium or myocardial disease.
identify specific pathology-related changes of echogenicity (18), results to date have generally been disappointing. Thus, echocardiography can be considered the modality of choice for the initial diagnostic workup, but has limited value for a more detailed disease myocardium or myocardial disease.
Computed tomography has been applied to assess left ventricle (LV) function and mass (19,20 and 21); its value for advanced tissue characterization however is limited, since there are no relevant applications of CT for markers for myocardial inflammation and edema, iron, amyloid, or diffuse fibrosis. Furthermore, CT is limited by radiation and the necessity for contract agents.
CARDIOVASCULAR MAGNETIC RESONANCE IN CARDIOMYOPATHIES: GENERAL ASPECTS
CVMR noninvasively and accurately visualizes left and right ventricular morphology and function (22,23). It is considered the in vivo gold standard for phenotyping patients with suspected or known cardiomyopathies.
As a unique feature of CVMR, it allows obtaining information on tissue pathology in cardiomyopathies. Since proton relaxivity depends on the chemical environment, pathologic processes with a more or less distinct local chemistry may allow a specific identification of diseased tissue. Consequently, disease-specific standardized protocols have been proposed (24).
CARDIOVASCULAR MAGNETIC RESONANCE APPROACH TO THE PATIENT WITH CARDIOMYOPATHY
MORPHOLOGY AND FUNCTION
Cardiomyopathies are characterized by specific alterations of ventricular and myocardial geometry, and/or function. To assess volumes and mass, generally white blood steady-state free precession (SSFP) gradient-echo sequences are applied during a breath-hold with approximately 30 phases per heartbeat. A stack of short-axis slices covering the entire LV from the mitral plane to the apex can be considered the gold standard to assess left ventricular volumes and mass (25,26). Recent developments in parallel imaging allow complete left ventricular coverage in one breath-hold but are not yet considered routine (27,28 and 29). Under routine clinical circumstances, a biplanar approach (long- and shortaxis views) may be sufficient (30,31) to estimate mass and systolic function. To cover the whole diastolic phase, techniques have been developed with continuous data acquisition and retrospective gating. The inclusion of the end-diastolic phase is important for the analysis of time-volume curves with respect to late diastole (32). Reliable angulation of the images by a series of angulated scouts obtained during breath-hold is crucial because the anatomic axis of the heart is not perpendicular to any of the orthogonal planes of the magnetic field. The slice thickness should be 8 to 10 mm; in case of circumscribed or subtle global changes, it should be reduced adequately. It is important to notice that there is a substantial shortening of the ventricular long axis (33) leading to a smaller number of slices covering the heart in systole compared with diastole. Commercially available software for automated edge detection may facilitate the evaluation process in clinical routine (34,35), but is so far not capable of including papillary muscles and may, therefore, not be sufficient for precise measurements.
A frequent finding in patients with cardiomyopathies is mild-to-moderate mitral regurgitation. Promoting factors are dilatation of the mitral valve ring (DCM, infiltrative cardiomyopathies) and papillary muscle dysfunction caused by infiltration (sarcoidosis, amyloidosis, hemochromatosis, or tumor). If quantification of mitral regurgitation is required for therapeutic decision making, an established technique using flow analysis should be performed (36). “Eyeball” quantification of the regurgitant jet, as used in ventriculography or echocardiography (37), may be misleading and should be used with great caution, in particular because the application of SSFP sequences results in a greater homogeneity of the blood pool and might therefore induce underestimation of valvular turbulences.
TISSUE
“Black blood” T1-weighted spin-echo techniques are often used for cardiac anatomy because of the excellent contrast between the myocardium and adjacent structures such as epicardial fat and intracavital blood. Slice orientation depends on the question being posed to the MR study; however, the orientation should include views orthogonal to the anatomic axis of the heart. Gadolinium (Gd) administration followed by a repeat T1 study may be helpful in infiltrative and inflammatory myocardial disease. T2-weighted image quality has markedly improved with short T1 inversion recovery (STIR) techniques, and fluid accumulation, such as edema and effusion in inflammatory or malignant diseases, may be sensitively visualized. Visualization of intramyocardial fibrosis is certainly desirable for the workup of cardiomyopathies. Newer T2-weighted protocols may improve overall image quality (38). Beyond imaging focal lesions, the quantification of absolute T1 or T2 is a reliable marker to detect diffuse myocardial changes (39,40,41 and 42). T1 mapping has recently emerged as a powerful tool for clinical assessment of myocardial diffuse fibrosis, further building on information already provided by late gadolinium enhancement (LGE)-CVMR. The prospective use of T1 mapping will provide much more precise myocardial tissue characterization (43), such as the quantification of diffuse myocardial fibrosis in heart failure (44), as well as use in the detection of acute myocardial edema (45). T2 mapping may provide a better delineation in the extent of disease when compared to signal-intensity-based methods (46,47).
METABOLISM
Magnetic resonance spectroscopy (MRS) has generally relied on 1H and 31P and has been applied in several studies of cardiomyopathy. Changes of high-energy phosphates as studied by 31P-MRS in cardiomyopathy were reported for DCM (48,49) and HCM (50). However, MRS remains an experimental approach for several reasons: 1H-MRS is
limited by a strong signal from water-bound protons and difficulties in spectral interpretation, and 31P-MRS is limited by the weakness of the phosphorus signal. Thus, voxels must be sufficiently large to cover circumscribed myocardial regions, and spectra are often altered by blood or adjacent tissue (e.g., skeletal muscle). Newer techniques feature irregularly shaped voxels and a significantly lower degree of spectral contamination (51). This approach may allow reproducible acquisition of reliable and highly informative myocardial spectra and even identify local pathology. Buchthal et al. (52) demonstrated changes in the ratio of myocardial phosphocreatine to adenosine triphosphate in women with chest pain but normal angiograms. However, MRS techniques require extensive experience and are prone to motion artifacts (53). Thus, the number of centers with access to this promising tool is currently limited.
limited by a strong signal from water-bound protons and difficulties in spectral interpretation, and 31P-MRS is limited by the weakness of the phosphorus signal. Thus, voxels must be sufficiently large to cover circumscribed myocardial regions, and spectra are often altered by blood or adjacent tissue (e.g., skeletal muscle). Newer techniques feature irregularly shaped voxels and a significantly lower degree of spectral contamination (51). This approach may allow reproducible acquisition of reliable and highly informative myocardial spectra and even identify local pathology. Buchthal et al. (52) demonstrated changes in the ratio of myocardial phosphocreatine to adenosine triphosphate in women with chest pain but normal angiograms. However, MRS techniques require extensive experience and are prone to motion artifacts (53). Thus, the number of centers with access to this promising tool is currently limited.
The increasing use of higher field strengths such as 3 T in recent trials will lead to the development of CVMR spectroscopy tools with high spatial resolution.
DILATED CARDIOMYOPATHY
DCM is characterized by a progressive dilatation of the heart with loss of contractile function (54). The typical pattern may be the final result of a disease process with multiple possible triggers, such as infectious organisms, toxic agents like anthracyclines, autoantibodies, or genetic disorders. The histologic hallmark of DCM is a progressive interstitial fibrosis with a numeric decrease of contractile myocytes. In advanced stages, DCM is also associated with at least relative wall thinning.
CARDIOVASCULAR MAGNETIC RESONANCE
CVMR nowadays recognized as a gold standard for functional imaging and assessment and with the state-of-the-art sequences is considered appropriate for many clinical indications (55). Main targets of CVMR studies in DCM are LV morphology and function using SSFP gradient-echo sequences (Fig. 10.1). CVMR has been proven to have low interobserver and intraobserver variabilities of left ventricular mass and volume measurements (56,57) with a good correlation to results obtained with positron emission tomography (58). CVMR was also used to analyze wall thickening in DCM (59), visualize impaired fiber shortening (60), and calculate end-systolic wall stress, which may be a very sensitive parameter for changes of LV function (61). The right ventricle (RV) is also frequently affected in DCM, and its morphology and function are accurately and reproducibly assessed by CVMR (62,63).
CVMR is the method of choice for a longitudinal followup in patients with DCM under pharmacologic interventions (64), and its reproducibility allows for a substantial reduction of the required sample size of clinical trials in DCM (65). Thus, costs could be reduced markedly and time could be saved in clinical research.
CVMR has also been shown to successfully detect fibrotic patterns, which allow for differentiating dilated from ischemic cardiomyopathy (66). A specific pattern involving intramural and especially subepicardial areas is often found in patients with myocarditis (Fig. 10.2). T1 mapping offers a completely new approach to detect diffuse myocardial fibrosis in patients with DCM, with a remarkable strong relationship with diastolic function and symptomatology (67).
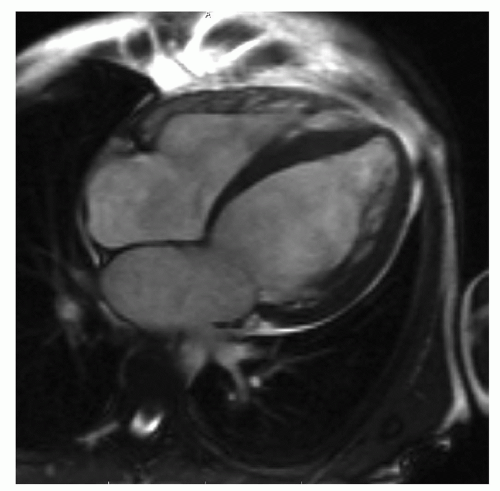 Figure 10.1. Diastolic steady-state free precession (SSFP) image in a patient with severe left ventricular dilatation and dysfunction in dilated cardiomyopathy. |
Recently, CVMR has shown the ability to accurately detect immunohistologically confirmed myocardial inflammation in patients with DCM (68).
LGE-CVMR is a strong and independent predictor of allcause mortality, independent of contractile function (69).
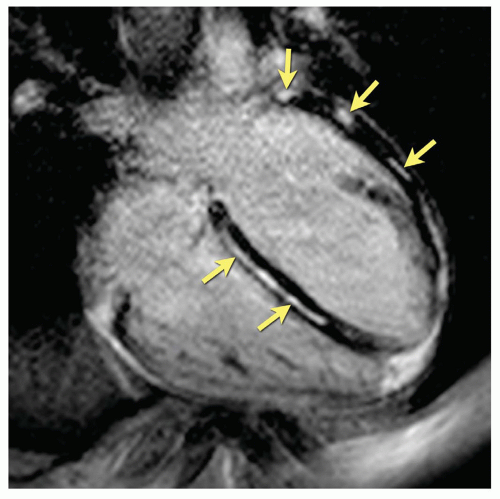 Figure 10.2. Inversion-recovery prepared gradient-echo image (“delayed enhancement”) in a patient with recent myocarditis showing focal necrosis of typical subepicardial localization (arrows). |
Spectroscopic studies have shown that high-energy phosphate metabolism is altered in DCM (70), and a low ratio of phosphocreatine to adenosine triphosphate as assessed by MRS was shown to be of prognostic value in DCM. Recent data indicate a role in the early detection of metabolic changes of the myocardium (71). Future studies will shed more light on this exciting field of research, and further clinical studies are warranted.
MYOCARDITIS
Myocarditis, caused by infectious agents, toxicity, or autoimmune processes, may be an important pathophysiologic component of cardiomyopathies including DCM (72) and ARVD/C (73). Common features of myocarditis include cellular infiltration, edema, necrosis, and fibrotic scars in late stage (74).
While echocardiography is still performed in cases of myocarditis to qualitatively assess functional abnormalities, wall thickness, and pericardial effusion (75), its diagnostic value may be limited in less severe cases, where function typically is normal.
One of the main advantages of CVMR over other imaging modalities is its ability to assess changes in tissue. In myocarditis, this has been used to assess edema, capillary leakage, hyperemia, and in severe cases, cellular necrosis and fibrosis (76). T2-weighted imaging, utilizing triple inversion-recovery turbo spin-echo sequences with inversion pulses for fat and blood suppression, has shown tremendous contrast for the differentiation of edema from myocardium (77). This technique has displayed high diagnostic accuracy for acute inflammatory or ischemic injury, which is significant as edema is an important hallmark of inflammatory injury (78,79 and 80). Signal-to-noise ratio of T2-weighted images still remains an issue but newly developed sequences may lead to better image quality and diagnostic accuracy (81). Contrast-enhanced fast spin-echo T1-weighted CVMR has also been used to assess hyperemia and capillary leak in myocarditis (82). This is done using an early gadolinium enhancement ratio (EGEr) to observe the distribution of contrast in the early distribution period. As opposed to EGE, Late Gadolinium Enhancement (LGE) of the myocardium specifically indicates irreversible injury, due to necrosis and fibrosis, thus making it an important diagnostic tool for late stages of myocarditis (74) (Fig. 10.3). Clinical studies have demonstrated that LGE has a high specificity for detection of irreversible injury in patients with myocarditis (Fig. 10.3) (78,83,84,85 and 86).
CVMR has become the primary diagnostic tool for assessment of myocardial inflammation in patients with suspected myocarditis (74). In 2006, The International Consensus Group on CVMR Diagnosis of Myocarditis was founded in order to achieve a consensus amongst CVMR experts to develop and standardize diagnostic protocols for CVMR in myocarditis. One of the significant conclusions reached by this consensus group was for the comprehensive use of CVMR criteria. Concluding that, based on two studies conducted comparing all three tissue-based techniques (75,76), presence of at least two positive criteria out of three is to be defined as a positive CVMR study for myocarditis (74).
Persisting CVMR markers for capillary leaks and hyperemia have been associated with reduced functional recovery (87). Edema as studied by T2-weighted CVMR was associated with a transient increase of LV mass in myocarditis (88).
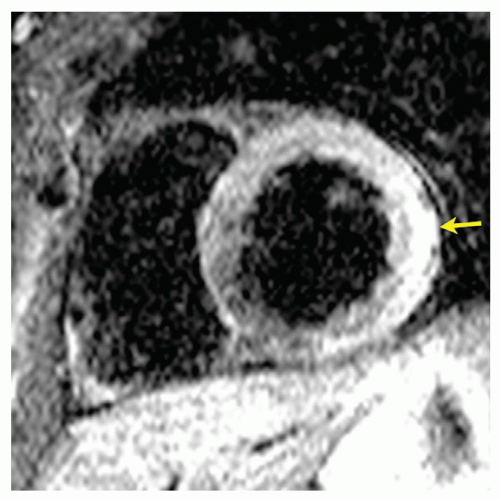 Figure 10.3. Short T1 Inversion Recovery (STIR) spin-echo image of myocardial edema in myocarditis (arrow). |
The ability to identify several tissue markers for inflammation or inflammatory damage renders CVMR the most comprehensive noninvasive diagnostic modality for myocarditis (74).
HYPERTROPHIC CARDIOMYOPATHY
HCM is classified as an autosomal dominant disease of the cardiac sarcomere and is characterized by generalized or localized left ventricular hypertrophy (92). Histologically, areas of hypertrophy reveal a pattern of myofibrillar disarray, which are not exclusively but predominantly seen in HCM and patchy areas of necrotic tissue caused by relative coronary insufficiency. HCM features inappropriate myocardial hypertrophy with loss of diastolic function. Almost two-thirds of patients with HCM show a Left ventricular outflow tract (LVOT) obstruction (93,94).
Endomyocardial biopsy is mainly performed for investigative reasons such as analysis of gene expression. However, for clinical decision making, the sensitivity and specificity in terms of exclusion of RCM and pressure-induced hypertrophy are not satisfying for an invasive procedure with inherent risks.
CVMR studies have been applied to assess mass, function, morphology, tissue characterization, and hemodynamic relevance of obstruction. Because of its high sensitivity for detecting regional morphologic changes and its noninvasive character, CVMR may be of special importance in screening families of index patients. In addition, there is evidence
showing the ability of CVMR to predict spontaneous clinical arrhythmias and sudden cardiac death (92).
showing the ability of CVMR to predict spontaneous clinical arrhythmias and sudden cardiac death (92).
CVMR cine sequences are suitable for functional studies, visualization of turbulent flow in LVOT obstruction, and mass quantification. In severe forms, the contracting ventricular walls may oppose each other and the end-systolic volume may remain less than 10 mL. Thus, a very careful contour definition and a contiguous set of short-axis slices are necessary to prevent underestimation of end-systolic volume, which is likely to occur when only long-axis views are used.
Mitral valve regurgitation, probably caused by a pathologic change of leaflet geometry, occurs frequently and should be included in a CVMR workup.
Diastolic function (or dysfunction) is a powerful clinical and prognostic factor in hypertrophy but does not yet belong to the routine measurements of CVMR. Preliminary clinical results suggest that tagging analysis of the early untwisting motion of the apical myocardium may be a helpful tool to assess diastolic dysfunction in hypertrophic heart diseases (95




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


