INTRODUCTION
Coronary artery disease (CAD) is a highly prevalent condition in the industrialized countries. About 7% of adults in the United States are estimated as being affected by CAD and a substantial increase is foreseen in next decades (
1). CAD is associated with a high morbidity and mortality, resulting in a major burden for the individual patient, for the health care system, and for the society (
1).
Primary prevention of CAD with management of the cardiovascular risk factors is the main component of disease control. However, once CAD is present, risk stratification is important for a tailored and efficient treatment (
2,
3). Determining the prognosis in CAD is complex. In addition to the specific cardiac situation, age, comorbidities, and variation in individual disease progression are important factors influencing the outcome of patients with CAD. Several aspects of the cardiac pathophysiology, which are important for risk stratification, can be assessed with cardiac imaging modalities. The severity of myocardial ischemia is one of the most important risk predictors and therefore represents a therapeutic target in CAD. In patients who already suffered a myocardial infarction (irreversible end-organ damage), the extent and the composition of the myocardial scar, as well as its functional impact on the cardiac performance, are important additional prognostic factors. Therefore, an accurate detection of myocardial ischemia and scar tissue, as well as a precise quantification of the cardiac function are important for the risk stratification and the treatment guidance of patients with CAD.
Cardiovascular magnetic resonance imaging (CMR) has emerged as a pivotal technique in the arena of cardiovascular imaging as an alternative, complementary, and frequently superior imaging modality, allowing for a comprehensive evaluation of all the above-mentioned aspects, without exposure to iodinated contrast agents or ionizing radiations. This chapter will provide an overview about the present role of CMR for the risk stratification of patients with CAD.
LEFT VENTRICULAR SYSTOLIC FUNCTION
The left ventricular ejection fraction is a quantitative measurement of the global left ventricular systolic function, and it is a well-known parameter for risk stratification of patients with CAD, since cardiovascular mortality, but also more specific outcomes such as malignant ventricular arrhythmias and sudden cardiac death are tightly related to it (
4,
5 and
6). Several mechanisms may cause left ventricular systolic dysfunction in patients with CAD, including: (
1) Myocardial infarction (scar tissue), (
2) myocardial hypo-perfusion (myocardial stunning and/or hibernating myocardium), and (
3) negative postinfarction remodeling process. Myocardial stunning is a reversible myocardial contractile abnormality caused by single or repetitive brief episodes of myocardial ischemia. Hibernating myocardium is caused by a persistent hypoperfusion, which is not severe enough to cause myocardial cell death, but is sufficient to cause contractile dysfunction (
7). Both forms are reversible after restoration of myocardial perfusion, but weeks may ensue before functional recovery occurs. Postinfarction remodeling is a process characterized by a progressive maladaptation of the myocardial tissue after an acute ischemic myocardial injury, causing fibrosis, apoptosis, and myocyte hypertrophy. This remodeling process is mainly driven by overstimulation of the neuroendocrine system and contributes to progressive
ventricular wall thinning, dilation, and functional compromise even in noninfarcted myocardium (
8). In a clinical perspective, the left ventricular ejection fraction is a “surrogate” of these different mechanisms of disease and therefore represents a “simple” and powerful parameter for risk stratification and patient selection for specific medical therapies or device implantation, such as internal cardiac defibrillator (ICD) or cardiac resynchronization therapy (CRT) (
4,
5). So, for example, a primary prophylactic ICD implantation after myocardial infarction is recommended in case of persistently reduced left ventricular ejection fraction ≤35% in patients in New York Heart Association (NYHA) class II-III according to the most recent guidelines (
4,
5). Therefore, a precise quantification of the left ventricular ejection fraction is of utmost importance, since it is a major determinant of prognosis and clinical decision making.
A detailed description of the technical aspects about the quantification of the ventricular systolic function by CMR is extensively reviewed in
Chapter 6. The ability to obtain images in all plane directions, the good temporal and spatial resolutions, the reliable endocardial border detection, and the independency of geometrical assumptions for the quantification of the ventricular volumes are main advantages of CMR, allowing for a more robust and less observer-variable quantification of ejection fraction as compared to other imaging modalities. Therefore, CMR is now recognized as the gold standard method for the quan-tification of the ventricular systolic function, and a precise quantification of the left ventricular volume and ejection fraction should be performed in every patient evaluated for CAD (
9).
RIGHT VENTRICULAR SYSTOLIC FUNCTION
Even though a myocardial infarction mainly involves the left ventricle, the right ventricle too may be affected by an ischemic injury, especially in case of occlusion of the right coronary artery, which usually supplies the majority of the right ventricular myocardium. In the acute setting, an extensive ischemic injury of the right ventricle is associated with a poor prognosis and needs specific treatment (
10). However, thanks to the lower oxygen consumption of the right ventricular myocardium and the direct oxygen diffusion from the ventricular cavity, the likelihood of functional recovery after an acute ischemic injury is higher as compared to the left ventricle (
11). Considering the complex geometry of the right ventricle, CMR offers clear advantages for the quantifi-cation of the right ventricular volumes and ejection fraction as compared with other imaging techniques. This is particularly true for two-dimensional echocardiography.
Multiple CMR studies provided the evidence that persistent right ventricular dysfunction and/or necrosis of the right ventricular myocardium after acute myocardial infarction are independent predictors of mortality and cardiovascular events, underscoring the importance of the assessment of the right ventricle for the global risk stratification of patients suffering an acute myocardial infarction (
12,
13).
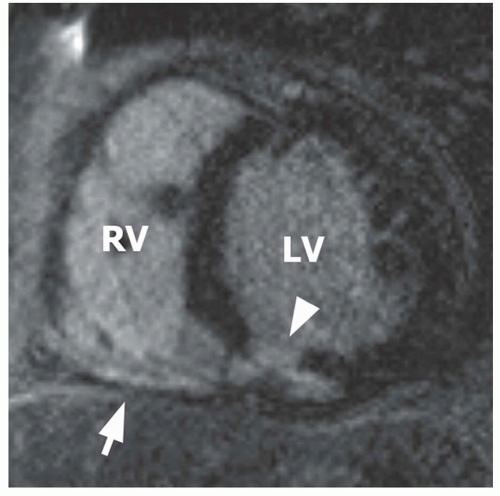
Figure 19.1 shows a typical example of inferior myocardial infarction, involving the basal aspect of the right ventricle.
MYOCARDIAL ISCHEMIA
In the presence of a hemodynamically significant coronary stenosis, the ability of increasing the myocardial blood flow on stress conditions is reduced, resulting in an imbalance between the demand and the delivery of oxygen and nutrients to the myocardial tissue, causing metabolic abnormalities of the cardiomyocytes with a consequent impairment of the contractile function. The assessment of myocardial ischemia by CMR is performed with two main techniques: (
1) Myocardial perfusion imaging and (
2) stress-induced myocardial wall motion imaging, which are extensively reviewed in
Chapters 15 and
16, respectively. Myocardial perfusion imaging evaluates the “first step” of the myocardial ischemic cascade, whereas stress-induced wall motion imaging evaluates the functional consequences of an insufficient perfusion on myocardial contractile performance. Considering that a physical stress test is not easily performable in a CMR scanner environment, the stress test is typically performed with positive chronotropic drugs (dobutamine) for myocardial wall motion imaging, and with vasodilator drugs (dipyridamole, adenosine, or regad-enoson) in case of myocardial perfusion testing. Both techniques are supported by a large body of evidence, showing a high diagnostic accuracy for the detection of hemody-namically significant CAD in a pooled meta-analysis of multiple studies evaluating the diagnostic performance of stress CMR (sensitivity and specificity of 83% to 86% for wall motion imaging and 91% to 81% for myocardial perfusion imaging, respectively) (
14). Here too, myocardial perfusion imaging was shown to provide a better diagnostic accuracy as compared to myocardial perfusion SPECT in multiple large comparative multicenter studies, underscoring
the robustness and the validity of this technique (
15,
16). Furthermore, the diagnostic performance and the prognostic value of the different CMR stress test modalities were proven in several clinical scenarios and in several patient populations, including patients suspected of CAD (as a test to role out CAD), patients with known CAD, patients with prior myocardial revascularization (including coronary artery bypass grafting (
17,
18) and percutaneous coronary angioplasty (
16,
17)), and patients presenting at the emergency department with acute chest pain and negative cardiac biomarkers (
19,
20 and
21). Consistently, patients with negative myocardial stress test (without evidence for myocardial ischemia) had an excellent cardiac prognosis with a low cardiovascular event rate during the follow-up (
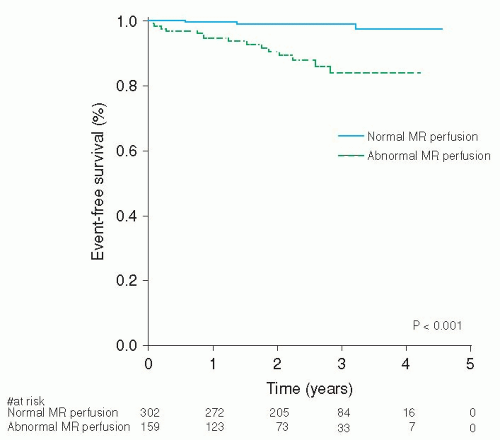
20,
21,
22,
23,
24,
25,
26,
27,
28 and
29) (
Fig. 19.2). Conversely, patients with evidence of myocardial ischemia have a worse cardiac prognosis. This also seems true for patients with prior myocardial infarction, where the presence and the extent of myocardial ischemia provide additional prognostic information (
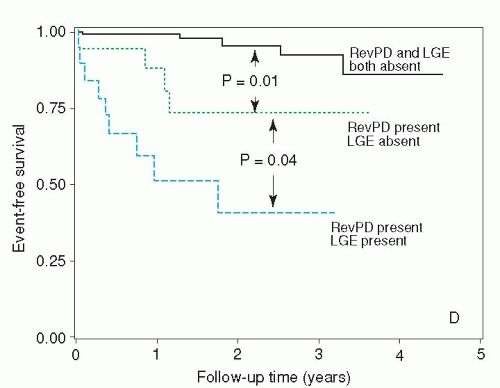
30,
31 and
32) (
Fig. 19.3). Notably, patients with prior myocardial infarction are exposed at a higher risk of malignant ventricular arrhythmias, because the myocardial scar represents the morphologic substrate for ventricular tachycardia. On top of that, ischemia of the peri-infarct zone may further destabilize the electrical membrane stability of the cardio-myocytes and therefore acts as an important trigger of such malignant arrhythmias.
Importantly, myocardial ischemia testing with an imaging modality enables identifying the localization and the extent of the ischemic myocardium. Indeed, all segments of the left ventricle are represented in both CMR perfusion and stress-induced myocardial wall motion imaging.
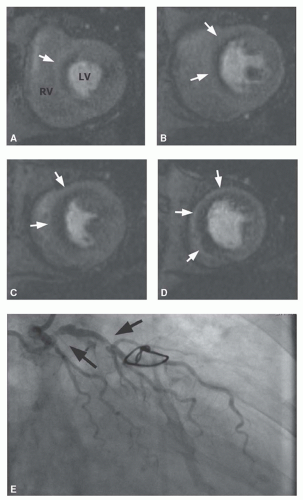
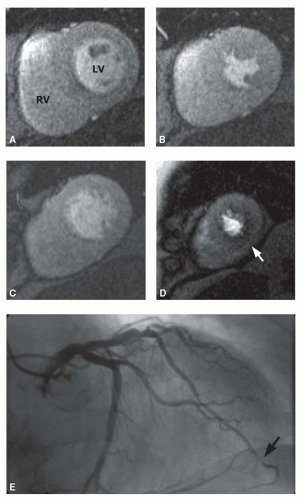
Figure 19.4 shows an example of an extensive ischemia involving the entire anterior and septal left ventricular myocardium, related to a severe stenosis in the proximal left anterior descending coronary artery.
Figure 19.5 demonstrates a small ischemia located in the inferior aspect of the ventricular apex, related to a stenosis of the distal left anterior descending coronary artery with a transapi-cal course. These two examples illustrate that CMR allows for a quantitative assessment of the ischemic myocardium. This is a central aspect, because the severity and the extent of myocardial ischemia are powerful outcome predictor in patients with CAD, and there is a rising evidence that the prognostic benefit of revascularization procedures among patients with stable CAD may depend on the extent of ischemic myocardium (
33,
34 and
35). In fact, in the current era where potent antianginal, lipid-lowering drugs, and anti-platelet therapies are available for secondary prevention of CAD, the results of multiple studies suggested that a treatment with myocardial revascularization is not superior to an optimal pharmacologic therapy in patients with mild-to-moderate stable CAD (
36,
37). However, large retrospective data and post hoc analysis of randomized trials suggested that myocardial revascularization had greater survival ben-efit as compared with medical therapy alone in patients with a severe inducible ischemia, suggesting that the extent of myocardial ischemia as assessed with a myocardial stress test may be the key component to identify low-risk patients, who can safely be treated medically, from high-risk patients, who may benefit from coronary angiography and revascularization procedure (
33,
34 and
35). The concept of ischemia-guided revascularization was also tested in a large multicenter trial, where patients undergoing percutaneous coronary intervention for multivessel CAD were randomly allocated to angiography-guided revascularization (where
coronary arteries with a stenosis >50% were treated), or ischemia-guided revascularization, where the hemody-namic significance of the coronary stenosis was evaluated invasively by measuring the fractional flow reserve. In line with the above-mentioned results, this study demonstrated that “functional,” ischemia-guided therapy was associated with a better cardiac prognosis and cost-effectiveness as compared with a “morphologic,” coronary angiogra-phy-guided therapy (
38,
39). All together, the functional evaluation of the coronary arteries by the different available stress tests is gaining a wide acceptance, and the most recent guidelines recommend to perform a functional stress test before a coronary invasive procedure in patients with stabile CAD, preferably using noninvasive testing before invasive angiography (
40).
Notably, the presence and the extent of myocardial ischemia adds incremental prognostic value as compared to the clinical profile, the left ventricular systolic function at rest, and the presence of myocardial scar (
30,
32). Therefore, CMR stress testing represents a valuable diagnostic tool for the initial evaluation and the surveillance of patients with known or suspected CAD, as it allows for a reliable
detection/role out of significant CAD, but also for monitoring of disease progression and risk stratification.
Tables 19.1 and
19.2 provide an overview of the literature about the prognostic value of CMR ischemia testing.