Fig. 17.1
Reformatted coronary MRI of the left coronary system acquired using a targeted free breathing acquisition with real time navigator gating and tracking in a healthy adult subject. The transverse acquisition displays the left main (LM), left anterior descending (LAD) and the left circumflex (LCX) coronary arteries. The in-plane spatial resolution is 0.7 × 1.0 mm2
Table 17.1
Diagnostic accuracy of ECG-triggered, free-breathing, targeted 3D and whole-heart coronary MRI with and without contrast agents
Study | Single-center/multi-center | # patients | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
Non-contrast 3D targeted coronary MRI | ||||
Kim et al. [10] | Multi-center | 109 | 88–98 | 32–52 |
Bunce et al. [30] | Single-center | 46 | 50–89 | 72–100 |
Sommer et al. [31] | Single-center | 107 | 74–88 | 63–91 |
Bogaert et al. [32] | Single-center | 21 | 85–92 | 50–83 |
Non-contrast 3D whole-heart coronary MRI | ||||
Jahnke et al. [33] | Single-center | 21 | 79 | 91 |
Sakuma et al. [27] | Single-center | 39 | 82 | 91 |
Sakuma et al. [26] | Single-center | 131 | 82 | 90 |
Pouleur et al. [34] | Single-center | 77 | 100 | 72 |
Kato et al. [35] | Multi-center | 138 | 88 | 72 |
Contrast enhanced 3D whole-heart coronary MRI | ||||
Yang et al. [36] | Single-center | 62 | 94 | 82 |
Yang et al. [37] | Multi-center | 272 | 91 | 80 |
Both gradient echo (GRE) and steady state free precession (SSFP) sequences [38] have been used for targeted thin-slab 3D acquisitions. Thin-slab 3D targeted acquisition with a GRE sequence results in more homogenous blood pool signal, but is heavily dependent on the inflow of unsaturated protons [39]. If coronary flow is slow or stagnant, saturation effects will cause a local signal loss that is often relatively exaggerated as compared with the lumen stenosis. Compared to GRE sequences, SSFP provides intrinsically higher signal due to its balanced gradients and improved blood-myocardium contrast due to its T1/T2 weighting [40] with reduced sensitivity to inflow effects. Both GRE and SSFP have been used for targeted 3D coronary MRI, where both have shown similar diagnostic accuracy for CAD [40, 41]. For whole-heart non-contrast coronary MRI at 1.5 T, SSFP appears to be the sequence of choice due to its higher blood-myocardium contrast and superior inflow properties [39].
Even with these technical advances, clinical acceptance of coronary MRI remains challenging due to coronary artery motion, long scan times, limited spatial resolution, suboptimal signal-to-noise-ratio (SNR) and blood-myocardium contrast-to-noise-ratio (CNR). The technical challenges in coronary artery MRI is different than other cardiovascular magnetic resonance (CMR) acquisitions due to unique issues including: (1) small caliber of coronary arteries (3–6 mm diameter), (2) high level of tortuosity, (3) near-constant motion during both the respiratory and the cardiac cycles, and (4) surrounding signal from adjacent epicardial fat and myocardium.
Cardiac Motion
Bulk epicardial motion is a major impediment to coronary artery and vein MRI and can be separated into motion related to direct cardiac contraction/relaxation during the cardiac cycle and that due to superimposed diaphragmatic and chest wall motion from respiration. The magnitude of motion from each component may greatly exceed the coronary artery diameter, thereby leading to blurring artifacts in the absence of motion-suppressive methods.
To compensate for bulk cardiac motion, accurate electrocardiographic (ECG) synchronization with QRS detection is required, and vector ECG approaches are preferred [42]. Coronary motion has been characterized using both catheter based x-ray angiography [43, 44] and CMR [45–47] methods during the cardiac cycle. Both the proximal/mid right coronary artery (RCA) and the left anterior descending (LAD) coronary artery display a triphasic pattern (Fig. 17.2), with the magnitude of in-plane motion nearly twice as great for the RCA. Coronary motion is minimal during isovolumic relaxation, approximately 350–400 ms after the R wave, and again at mid-diastole (immediately prior to atrial systole). The LAD diastasis is longer than the RCA, and begins earlier in the cardiac cycle [12]. The duration of the mid-diastolic diastasis period is inversely related to the heart rate and dictates the coronary data acquisition interval.
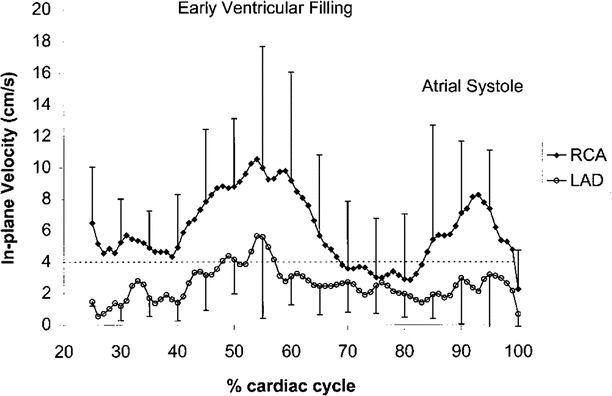

Fig. 17.2
Graph depicting the in-plane motion of the right coronary artery (RCA) and the left anterior descending (LAD) coronary artery during the cardiac cycle. The x-axis displays time as a percentage of the R-R interval. Note the image quality of the RCA cross section improves when the acquisition is performed during mid-diastole as compared to early diastole (Reproduced from [47], with permission of Wiley)
For coronary artery MRI, the acquisition interval is adapted to the heart rate/diastasis interval using a patient-specific diastasis period. This can be readily identified by the acquisition of high temporal resolution cine dataset orthogonal to the long axis of the proximal/mid RCA and of the LAD. Semi-automated tools to identify the optimal data acquisition window have also been proposed [48, 49]. For patients with a heart rate of 60–70/min, a coronary artery MRI acquisition duration of ~80 ms during each cardiac cycle results in improved image quality [14]. The duration must be further abbreviated (e.g., <50 ms) at higher heart rates, while with bradycardia, the acquisition interval can be expanded to 120 ms or longer. The use of patient-specific acquisition windows serves to reduce overall scan time [50, 51]. Image degradation can be caused by sinus arrhythmia, leading to heart rate variability, which is common especially in younger adults [52]. An adaptive real-time arrhythmia rejection algorithm can correct for heart rate variability, and improves coronary artery MRI quality [49].
Respiratory Motion
The second major challenge for coronary artery MRI is compensation for respiratory motion. With inspiration, the diaphragm may descend up to 30 mm and the chest wall expands – resulting in an inferior displacement and anterior rotation of the heart [53]. Several approaches have been proposed to minimize respiratory motion artifacts, including sustained end-expiratory breath-holding, chest wall bellows, respiratory navigators, fat navigators and self-gating methods.
Prolonged (15–20 s) end-expiratory breath holds were utilized to suppress respiratory motion in initial 2D coronary artery MRI methods [54]. Breath holding offers the advantage of relative ease of implementation in compliant subjects, but it limits the temporal acquisition window, image spatial resolution and anatomic coverage. Additionally, many patients are unable to adequately sustain a breath-hold. Furthermore, slice registration errors (due to variability in end-expiratory diaphragmatic position) are very common as is diaphragmatic drift during the breath hold [54–57] and may occur in up to half of patients [12]. Supplemental oxygen and hyperventilation (separately or in combination) can be utilized to prolong the breath-hold duration [56, 57], but these methods may not be appropriate for all patients, and both diaphragmatic drift and slice registration errors persist [57].
Diaphragmatic respiratory navigators, first proposed by Ehman [58] for abdominal MR imaging, enable free-breathing acquisitions without the stringent time constraints and patient cooperation requirements imposed by multiple breath holds, and thus offer superior spatial resolution opportunities. Although the specifics of navigator implementation varies among CMR vendors, in the ideal implementation, the navigator can be positioned at any interface that accurately reflects respiratory motion, including the dome of the right hemidiaphragm (Fig. 17.3) [59, 60], the left hemidiaphragm, the anterior chest wall, the anterior free wall of the left ventricle [60, 61], or even through the coronary artery of interest. The navigator should not cause an image artifact and should be temporally located immediately preceding the imaging portion of the sequence with data accepted (used for image reconstruction) only when the navigator indicates that the “interface” (e.g., diaphragm position) falls within a user-defined window. The dome of the right hemidiaphragm has become the preferred location [27, 38–40] due to the simplicity and ease in set-up, where the motion of the right hemidiaphgragm in the superior-inferior direction can be tracked. From CMR studies of cardiac border position during the respiratory cycle, Wang observed that at end-expiration, the ratio between cardiac and diaphragmatic displacement is ~0.6 for the RCA and ~0.7 for the left coronary artery [53] though there is variability among subjects [20, 42, 43] and position (e.g., supine vs. prone imaging) [23]. This rule-of-thumb offers the opportunity for prospective navigator gating with real-time tracking [60, 62], in which the position of the interface (diaphragm) is determined, and the slice position coordinates can then be shifted in real-time (before the data collection) to appropriately adjust spatial coordinates [63]. This technique allows for the use of wider gating windows and increased navigator efficiency, leading to shorter scan times. Real-time tracking implementation with a 5-mm diaphragmatic gating window is often used resulting in a navigator efficiency approaching 50 % [62]. Coronary artery MRI with real-time navigator tracking has been shown to minimize registration errors (as compared with breath holding) while maintaining or improving the image quality [12, 62]. It should also be noted that the quality of coronary artery MRI is improved by using consistent ECG timing as well as respiratory suppression methodology for both the coronary localizing/motion scout images and for the coronary artery MRI acquisitions [51].
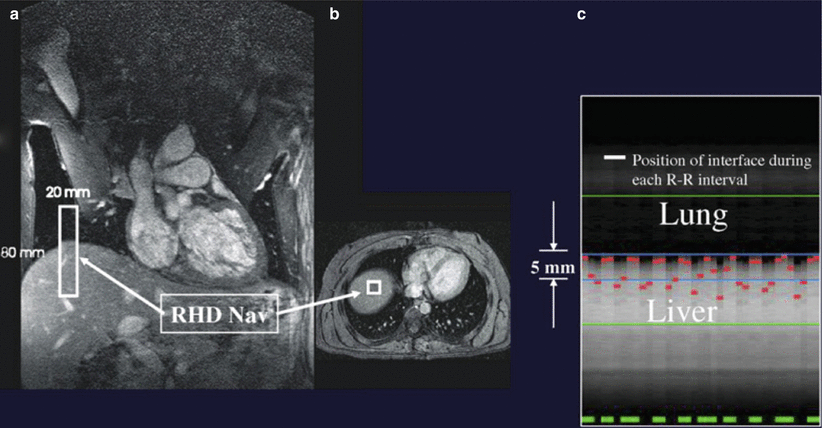

Fig. 17.3
Positioning and utility of the respiratory navigator. Coronal (a) and axial (b) thoracic images for placing the navigator at the dome of the right hemidiaphragm (RHD NAV). (c) Respiratory motion of the lung-diaphragm interface is recorded using a 2-D selective navigator with the lung (superior) and liver (inferior) interface. In this example, the maximum excursion between end-inspiration and end-expiration is ~11 mm. The position of the lung-liver interface at each R-R interval is indicated by the broken line in the middle of (c). Data are only accepted if the lung-liver interface is within the acceptance window of 5 mm. Data acquired with the navigator outside of the window are rejected. Accepted data is indicated by the broken green line at the bottom of (c)
A number of refinements to the navigator method have been proposed. While a “fixed” superior-inferior correction factor of 0.6 (with no left-right or anterior-posterior correction) [44, 45] is commonly used, significant individual variability has been observed [55]. A subject-specific tracking factor has been advocated and shown to improve the quality of coronary images when the subject-specific tracking factor differs from 0.6 [64]. The use of multiple navigator locations, use of leading and trailing navigators, and navigators that provide guidance for affine transformations, i.e. 3D translations and rotations, of the slice prescription for each heart-beat have been proposed [23, 65–67]. The affine transformation permits use of larger navigator windows, and hence higher navigator efficiency. It has also been proposed that the heart itself be tracked [20, 68–70]. For instance, methods that track the epicardial fat to detect the heart position have been proposed [71–74]. In addition to navigator gating, respiratory self-gating techniques have been investigated, a method that derive the respiratory position of the heart from the imaging data itself [19, 20, 28, 68, 69, 75, 76], thus avoiding certain issues with navigators such as subject-dependent tracking factor [77] and hysteresis effects [78]. Navigator with fixed scan efficiency has also been recently introduced which results in imaging at a fixed scan time [79]. Novel k-space trajectories and various image reconstruction based method such as cross-correlation of low resolution images have also been proposed for respiratory motion compensation [28, 80–82].
SNR and CNR
The coronary arteries are surrounded by epicardial fat and the myocardium. Thus contrast-to-noise ratio (CNR) can be improved by suppressing the fat and myocardium signal surrounding the coronary arteries. Frequency (spectrally) selective pre-pulses are applied to saturate signal from fat tissue, thereby allowing visualization of the underlying coronary arteries [54, 83]. To differentiate myocardium and the coronary lumen, endogenous contrast preparation techniques are commonly utilized [14, 83–86]. Two methods that can enhance the contrast between the coronary lumen and underlying myocardium are T2 preparation pre-pulses [14, 84, 85, 87] and magnetization transfer contrast [83, 86]. The former is often used for coronary artery MRI as it also suppresses deoxygenated venous blood, while the latter is used for coronary vein MRI [57].
The limited signal-to-noise ratio (SNR) in coronary artery MRI, along with constraints on acquisition duration, restricts the spatial resolution in the acquisition. Spatial resolution requirements for clinical coronary artery MRI depend on whether the goal is to identify the origin and proximal course of the coronary artery (e.g., issues of anomalous coronary disease), or whether the goal is to identify focal stenoses in the proximal and middle segments.
The SNR of coronary MRI can be enhanced by higher B0 field strength [88], larger 3D spatial coverage [29], vasodilator administration, and contrast agents based on gadolinium chelates. The intrinsically higher SNR associated with higher magnetic field strengths may be advantageous for non-contrast coronary MRI. However, additional considerations, such as higher B1 and B0 inhomogeneity and higher specific absorption rate, affect certain aspects of coronary MRI, such as the diminished utility of SSFP sequences at 3 T due to increased field inhomogeneity and high specific absorption rate. Hence, GRE sequences, which are less sensitive to field inhomogeneity, as well as localized shimming [89] and contrast preparation techniques that deal with B1 inhomogeneities [87, 90] have been advocated.
The increased coverage of whole-heart coronary MRI can potentially improve the SNR but this also increases the scan time. Thus, the SNR gain is often counteracted by the need for accelerated imaging to reduce scan time, which carries an SNR penalty. Despite this penalty, excellent image quality of whole-heart coronary MRI has been shown in several studies [26, 29], and an example from a single-center study [26] is depicted in Fig. 17.4. Furthermore, whole-heart imaging suffers from saturation effects of the inflowing blood magnetization [39]. Another technique to improve SNR in coronary MRI is the administration of vasodilators, since the increased coronary blood flow secondary to vasodilatation reduces the inflow saturation effects [91, 92]. Figure 17.5 demonstrates impact of sublingual isosorbide dinitrate administration on 3D targeted coronary MRI up to 30 min after drug administration in terms of subjective image quality and objective SNR and vessel sharpness.
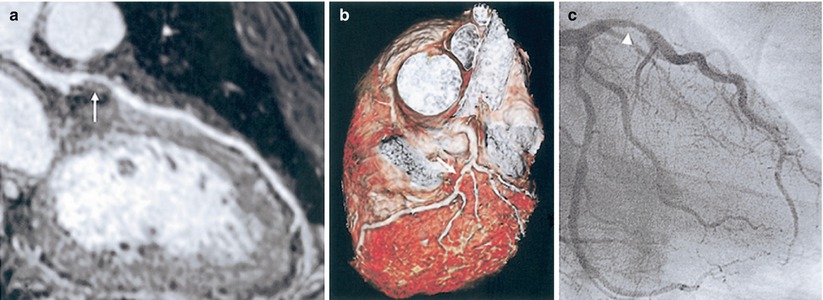
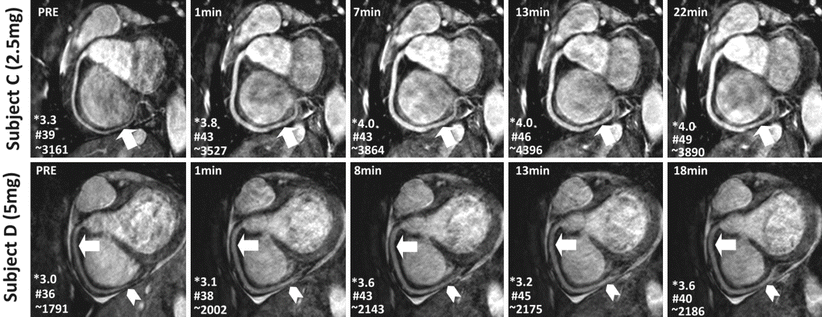

Fig. 17.4
Whole-heart coronary MRI. (a) A stenosis in LAD is visualized using a curved multiplanar reconstruction (white arrow). (b) A three-dimensional view of LAD with stenosis is depicted in the volume-rendered image. (c) X-ray coronary angiography confirms proximal LAD stenosis (arrowhead) (Adapted from Ref. [26], with permission of Elsevier)

Fig. 17.5
Reformatted images from a targeted 3D coronary MRI of the RCA acquired before and after sublingual isosorbide dinitrate administration on two healthy subjects using a 3D free-breathing SSFP with 2.5 mg (top row) and 5 mg doses (bottom row) as a function of time. Improved RCA vasodilation and signal enhancement can be observed in all images post-isosorbide dinitrate (arrows in top and bottom row). The enhanced SNR with isosorbide dinitrate also allows for improved visualization of the distal segments
The administration of exogenous gadolinium contrast agents (both extracellular [16, 93, 94] and intravascular [95–100]) that shorten the T1 relaxation time provides an alternative flow-independent approach to improve SNR and CNR. Since conventional extracellular contrast agents, e.g. gadopentetate dimeglumine (Gd-DTPA), diffuse rapidly into the interstitial space, early contrast-enhanced coronary MRI studies focused on breath-hold technique to take advantage of the first-passage of these agents [94]. However, both the breath-hold and first-pass aspects of such approaches limit the spatial resolution and is unsuitable for whole-heart coronary acquisitions [29]. Following the availability of a high relaxivity extracellular contrast agent, gadobenate dimeglumine (Gd-BOPTA; MultiHance; Bracco Imaging SpA, Milan, Italy), improved whole-heart coronary MRI at 3 T was shown to be feasible using a T1-weighted inversion recovery (IR) GRE sequence with a slow infusion of this Gd-BOPTA [16]. An example of contrast-enhanced whole-heart coronary MR image from a CAD patient and the corresponding x-ray angiogram are shown in Fig. 17.6, demonstrating agreement between two modalities in detecting significant stenosis. In a single center trial, 3 T whole-heart coronary MRA with slow infusion of Gd-BOPTA had 93 % sensitivity, 89 % specificity and 90 % accuracy for detecting >50 % diameter stenosis on a per-vessel basis (and 94 %, 82 % and 89 % on a per-patient basis) when compared with x-ray angiography [36]. A bolus infusion of Gd-BOPTA for coronary MRI has also been reported [101], and an example depicted in Fig. 17.7 shows a clear visualization of the three major coronary vessels in the reformatted and 3D volume rendered images. Furthermore, the bolus contrast injection method is advantageous in multiple ways, since it simplifies the initiation time of coronary MRI acquisition compared to slow infusion, and it is compatible with late gadolinium enhancement imaging, which enables the assessment of coronary artery stenosis and myocardium viability using a single bolus contrast injection.
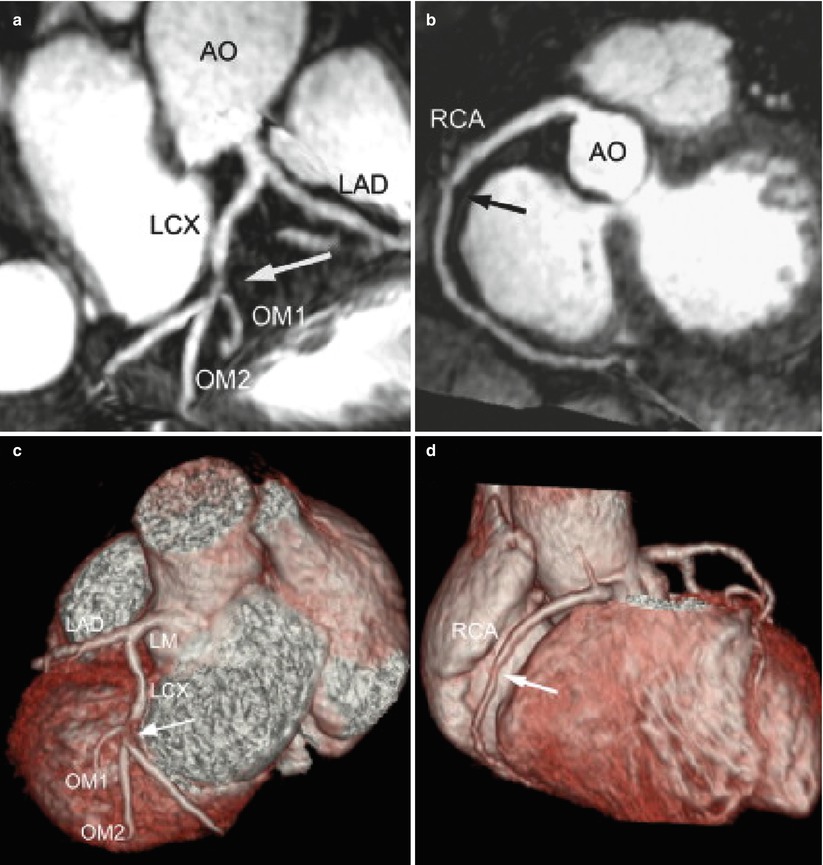
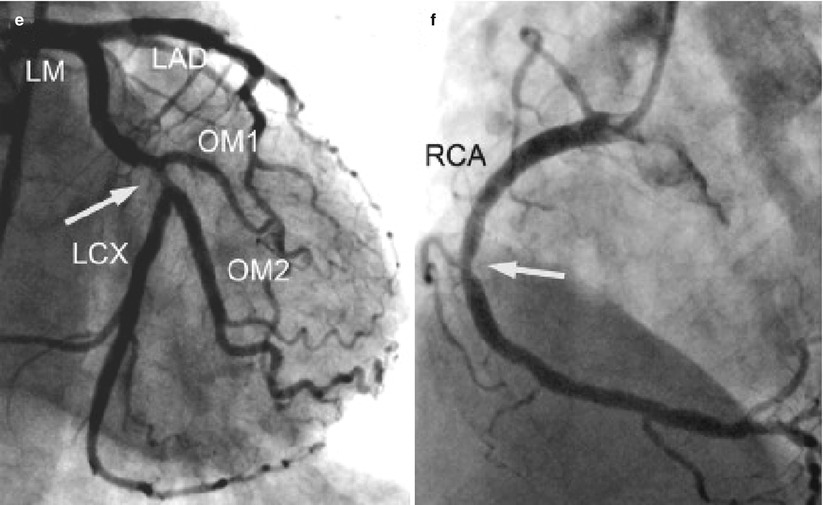


Fig. 17.6
Contrast-enhanced whole-heart 3D coronary MRI with a slow infusion of Gd-BOPTA contrast agent in a patient with atypical chest pain. (a, b) Contrast-enhanced whole-heart maximum intensity projection images show a significant stenosis in the proximal LCX and a non-significant stenosis in the middle RCA (arrows), respectively. (c, d) The volume-rendered images have the same findings in LCX and RCA (arrows). These were consistent with the findings (arrows) of conventional coronary angiography (e, f). AO aorta, OM obtuse marginal artery (Adapted from Ref. [36], with permission of Elsevier)

Fig. 17.7
Whole heart SSFP coronary MRI acquired with a bolus injection of Gd-BOPTA. (a) 3D volume rendering of the acquisition volume. (b) Corresponding reformatted whole-heart image. All three major coronary arteries and distal branches are clearly depicted. RCA right coronary artery, LAD left anterior descending, LCX left circumflex
Coronary MRI: Advanced Methods
The sensitivity and specificity of coronary MRI for detection of CAD remain moderate based on single-center [26, 27, 30–34, 36] (Table 17.1) and multi-center [10, 35, 37] studies, despite the tremendous technical improvements in the last two decades. Coronary motion, SNR and CNR remain as major impediments to coronary MRI, and these issues need to be addressed before clinical prime time for coronary MRI. To overcome some of these hurdles, several CMR centers continue with the development and implementation of novel approaches, including non-Cartesian acquisitions, accelerated imaging techniques, coronary vein MRI and higher field imaging.
Non-Cartesian acquisitions provide efficient k-space traversals that lead to incoherent or less visually significant artifacts. Thus, alternative non-Cartesian k-space acquisitions, including spiral and radial coronary MRI have received attention. The use of spiral coronary artery MRI was first reported by Meyer and colleagues [102]. Spiral acquisitions are advantageous to Cartesian acquisitions in several respects, including a more efficient filling of k-space, enhanced SNR [40, 103], and favorable flow properties. However, there are also drawbacks associated with spiral trajectories, such as increased sensitivity to magnetic field inhomogeneity and longer image reconstruction. Interleaved spiral imaging is typically used due to reduced artifacts [102–105], though a single-shot k-space trajectory can also be employed. Both breath-hold (2D) and free-breathing/navigator-gated acquisitions can be performed with spiral coronary imaging [40, 95, 103, 105]. Compared to conventional Cartesian approaches, single spiral acquisitions (per R-R interval) afford a near threefold improvement in SNR [40, 103]. Hence, acquiring two spirals during each R-R interval will halve the acquisition time, while maintaining superior SNR (vs. Cartesian acquisition) and CNR. Variable density spirals have also shown benefit [82].
Radial trajectories also enable more rapid acquisitions, while decreasing sensitivity to motion. Data in healthy subjects appear promising [40, 106–108] and may be particularly beneficial for coronary wall imaging [70, 109, 110].
Parallel imaging techniques such as generalized autocalibrating partially parallel acquisition (GRAPPA) [111] or sensitivity encoding (SENSE) [112] are the most commonly used clinical acceleration technique for coronary MRI [16, 36, 100, 101]. Resultant acceleration rates of up to twofold while using 5–16 element cardiac-coil arrays, and up-to fourfold acceleration rate using 32-channel coils have been achieved [24, 113]. Currently, parallel imaging is considered the state-of-the-art accelerated imaging technique for whole-heart coronary MRI, and is commonly utilized for clinical imaging.
In addition to the non-Cartesian trajectories [114] described previously, compressed sensing (CS) has emerged as an alternative acceleration technique that exploits the sparsity of the image in a transform domain [115, 116]. CS also requires an incoherent undersampling pattern, which can be achieved by random undersampling of k-space data in the ky-kz plane for three-dimensional (3D) Cartesian acquisitions. In high-resolution coronary MRI, an advanced CS-based reconstruction strategy was shown to provide reconstructions with reduced blurring compared to conventional CS techniques [117], and was successfully utilized in contrast-enhanced whole-heart coronary MRI [118]. More recently, for highly-accelerated sub-millimeter resolution whole-heart coronary MRI, CS was shown to outperform parallel imaging at sixfold accelerated imaging in a head-to-head comparison [119], with example images depicted in Fig. 17.8. CS can also be used in conjunction with non-Cartesian imaging, such as with spiral acquisitions to enable whole heart acquisitions in a single prolonged breath-hold [81] or with 3D radial trajectories [108].

Fig. 17.8
Example images from two separate highly-accelerated sub-millimeter resolution whole-heart coronary MRI. An example coronal slice (top) containing a cross-section of the left main (LM) shows that SENSE images, acquired with sixfold uniform undersampling (right), suffers from noise amplification. In contrast, the LM is clearly visualized using an advanced CS-based technique (LOST), acquired using sixfold random undersampling (left). In the reformatted coronal images (bottom), the proximal LCX cannot be tracked due to the high noise level in the SENSE reconstruction, but RCA and LCX branches are visualized with the LOST technique
Coronary MRI at high fields has been an active area of research, due to potential benefits in SNR and CNR. SNR is directly related to field strength (B0), and thus 3 T imaging would offer the opportunity to double SNR compared to 1.5 T systems [120]. While the vast majority of coronary artery MRI investigations have been performed on 1.5 T systems, clinical 3 T systems are increasingly available and becoming the platform of choice for testing of many advances.
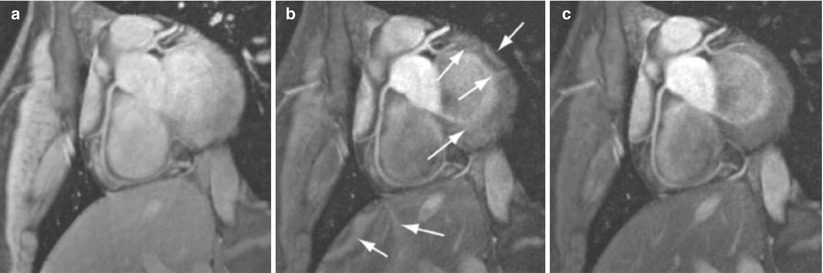
Technical challenges associated with 3 T coronary MRI include increased susceptibility artifacts, field inhomogeneities [87], reduced T2* [121, 122], increased specific absorption rate (SAR), T1 prolongation and the amplified magnetohydrodynamic effect [42]. At 3 T, free breathing navigator and breath-hold 3D coronary artery MRI studies in healthy volunteers have demonstrated >50 % improvement in SNR with impressive image quality using segmented k-space gradient echo or SSFP [123], as well as spiral and contrast enhanced methods [88, 124, 125]. Coronary MRI at 3 T using SSFP sequences is challenging due to increased field inhomogeneity and high SAR, thus GRE sequences have become widely used for coronary MRI at 3 T. To reduce the impact of B1 inhomogeneity at the high field strengths, improved preparation sequences such as adiabatic T2 magnetization preparation [87, 90] and adiabatic fat saturation have also been utilized. Figure 17.9 shows an example of coronary MR images acquired at 3 T using improved T2 magnetization preparation, which suppresses the banding artifact resulting from conventional T2 magnetization preparation. Despite these technical improvements, there are currently no multi-center data on a head-to-head comparison between 3 and 1.5 T coronary MRI for diagnosing CAD. Coronary MRI at even higher field strengths, such as 7 T [126], is even more challenging. Figure 17.10 shows an example coronary MRI from a healthy subject acquired at 7 T. Several technical issues, including coil design, motion compensation and B0 and B1 field inhomogeneity, need to be addressed before clinical evaluation is possible.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


