Lymphocytic Myocarditis
Fabio R. Tavora, M.D., Ph.D.
Allen P. Burke, M.D.
Pathogenesis
“Lymphocytic myocarditis” describes the histologic pattern of the most common form of myocarditis, which is associated with viruses and autoimmune responses. It is hypothesized that after viral entry, viral replication causes acute myocyte injury, myocyte necrosis, exposure of intracellular antigens, and activation of the immune system. After an acute phase of infiltration of natural killer cells, macrophages, and neutrophils, a second autoimmune phase is defined by activated virusspecific T lymphocytes, which may target the host’s organs by molecular mimicry. It is in this phase, when virus has largely been cleared, that diagnosis is generally made by biopsy or at autopsy.1
A causative link between viruses and myocardial inflammation is difficult to prove, because viral cultures and serology are often unrewarding, with low yields.2 A large number of studies that have utilized molecular techniques have investigated the presence of biopsies in tissue samples of patients with myocarditis and dilated cardiomyopathy. Because of the presence of transmembrane receptor CAR (coxsackievirus and adenovirus receptor) and the established murine model for coxsackievirus myocarditis, coxsackieviruses and adenoviruses were among the first viruses studied by polymerase chain reaction. Early studies based on tissue samples with acute myocarditis showed a high rate of positivity for viruses, especially adenoviruses, with confirmation by culture in a small subset of patients.2,3 Subsequent studies using polymerase chain reaction, primarily on endomyocardial biopsies, showed a smaller and widely variable number of samples with enteroviruses or adenoviruses, ranging from 1% to 60% (reviewed in Ref. 4).
Molecular techniques have greatly increased the scope of viruses detected in cardiac tissues, including cytomegalovirus, parvovirus, human herpes virus 6, and Epstein-Barr virus.4 There is an especially high rate of detection of HHV6 and parvovirus 19, which have been localized to macrophages and endothelial cells in the heart, respectively, leading to a “shift” in the focus of viral etiology away from adeno- and enteroviruses. Whether these agents are causative or “innocent bystanders” has yet to be determined, however.
Because an autoimmune reaction is believed to play a role in viral myocarditis, it is not unexpected that the histologic features are similar with those of myocarditis associated with rheumatologic autoimmune diseases (see Chapter 10).
Clinical Findings
Myocarditis is generally a disease of infants, children, and young adults. The most commonly implicated etiologies-entero- and adenovirusesare ubiquitous with widespread serologic immunity across the adult population. There is no known genetic predisposition to the development of myocarditis after viral infection; familial cases are rare,5 although families with dilated cardiomyopathy have been found to harbor antimyocardial antibodies.6
The clinical diagnosis is made on the basis of electrocardiographic changes, myocardial damage evidenced by elevated troponin, evidence of systemic inflammation, lack of epicardial coronary artery disease, and generally mild and transient ventricular dysfunction. Relatively uncommonly, there is severe ventricular dysfunction (“fulminant myocarditis”), which responds to steroids and other immunosuppressive therapies.7 Fulminant myocarditis is diagnosed on the basis of clinical features at presentation, including the presence of severe hemodynamic compromise, rapid onset of symptoms, and fever.
Most patients present with symptoms of concomitant pericardial inflammation, chest pain, and palpitations. Patients with the combination of myocarditis and pericarditis (myopericarditis) typically have chest pain, flu-like symptoms, and pericardial friction rubs, in addition to significant increases in serum troponins. Most patients respond to treatment with nonsteroidal anti-inflammatory drugs, and the prognosis appears to be better than that of pure myocarditis.8
Long-term follow-up studies in patients with acute myocarditis show that ˜20% develop dilated cardiomyopathy in 3 years.9
Coxsackie B viral myocarditis may occasionally present at birth and present with signs and symptoms of heart failure. Virus is detected in the blood, cerebrospinal fluid, nasopharyngeal swab, or stool by PCR or culture. The mortality is nearly one-third, and the survivors typically develop left ventricular aneurysms.10
Magnetic Resonance Imaging
The magnetic resonance changes in myocardial tissue during the first phase of myocardial inflammation include edema on T2-weighted images, which is generally consistent with inflammation. Late gadolinium enhancement imaging generally reveals an intramural, rim-like pattern in the septal wall or patchy subepicardial distribution in the left ventricular lateral free wall.1
Diagnosis of Myocarditis at Autopsy
Gross Findings
Grossly, myocarditis reveals nonspecific findings ranging from normal to dilatation of all four chambers, depending on the specific agents and the duration of illness. Ventricular softening and pallor is typical but not always evident. Fibrinous pericarditis can be associated with cases of myocarditis and does not necessarily indicate bacterial etiology. Fibrosis is a component of healed myocarditis, and the distribution is random, as opposed to subendocardial-based scars of ischemia.12 Scars that are subepicardial, as opposed to subendocardial or transmural, are rarely ischemic, unless secondary to embolization from coronary thrombosis. Subepicardial scars should raise the differential diagnosis of healed myocarditis or arrhythmogenic cardiomyopathy. Rare cases of diffuse calcification of the myocardium following myocarditis have been reported.13 Figure 163.1 shows a series of axial cuts on a case of myocarditis with the typical myocardium pallor and healed fibrosis.
Microscopic Findings
In cases of sudden death due to myocarditis, the infiltrate is generally diffuse, in all sections examined, and there is no question as to
diagnosis or mechanism of death, which is attributed to ventricular arrhythmia (Fig. 163.2). There may be variability in the extent of inflammation from slide to slide, and endocardial as well as inflammation in the epicardial fat is not uncommon.
diagnosis or mechanism of death, which is attributed to ventricular arrhythmia (Fig. 163.2). There may be variability in the extent of inflammation from slide to slide, and endocardial as well as inflammation in the epicardial fat is not uncommon.
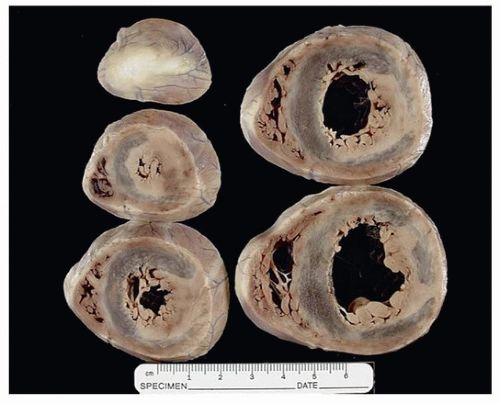 FIGURE 163.1 ▲ Myocarditis. A 4-year-old child died after complaining of weakness and shortness of breath; there was a history of recent diarrhea and vomiting. Short-axis sections of the heart show irregular mottling of the myocardium, corresponding to areas of hyperemia or lymphocytic infiltration. In most cases of fatal myocarditis, the heart appears grossly normal.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|