Chapter 65 Lung Cancer
Epidemiology, Surgical Pathology, and Molecular Biology
Epidemiology
Incidence and Survival
When the mortality rates (deaths due to disease within a calendar year) for lung cancer in women and men are plotted against the calendar years for the last 50 years, one notes a marked difference in the pattern of the curves (Figure 65-1). The incidence rates for men show a marked rise from the early 1950s to the beginning of the 1990s, whereas rates for women lag by about a quarter of a century and then show an identical rise, essentially parallel to those for men, and continuing into the 21st century. By the 1950s, death due to lung cancer far exceeded prostate and colon cancer deaths among men, and by 1990, lung cancer deaths among women exceeded breast and gynecologic cancer deaths. Usually cancer mortality follows cancer incidence; however, in the case of lung cancer, owing to the very high cancer fatality rate (mortality from cancer for persons given a diagnosis of cancer), lung cancer mortality exceeds the most common cancers in both men and women. As a result of the Surgeon General’s 1964 report on smoking, men began to stop smoking in the succeeding 20 to 30 years; the incidence and mortality of lung cancer began to decline from a peak in the early 1990s to a level that approximates the mortality rate of the 1970s. The phenomenon of social smoking among women lagged behind that for men; subsequently, both the incidence of lung cancer in women and its associated mortality continue to rise into the present day.
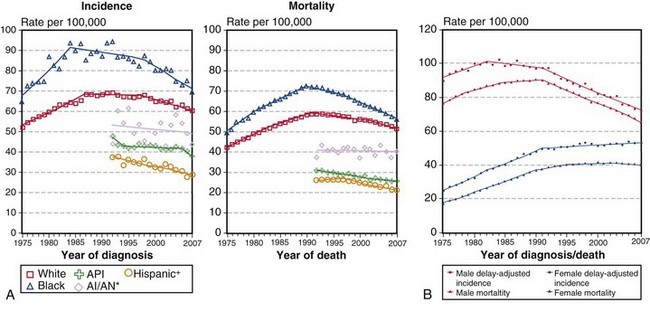
(A, Incidence data for whites and blacks are from the SEER nine areas—San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta. Incidence data for Asian/Pacific Islanders, American Indians/Alaska Natives, and Hispanics are from the SEER thirteen areas—the SEER nine areas plus San Jose–Monterey, Los Angeles, Alaska/Native Registry, and Rural Georgia. Mortality data are from U.S. mortality files, National Center for Health Statistics, Centers for Disease Control and Prevention [CDC]. Rates are age-adjusted to the 2000 U.S. standard population [19 age groups, Census P25-1103]. Regression lines are calculated using the Joinpoint Regression program Version 3.4.3, April 2010, National Cancer Institute. Joinpoint analyses for whites and blacks during the 1975-2007 period allow a maximum of 4 joinpoints. Analyses for other ethnic groups during the period 1992 to 2007 allow a maximum of 2 joinpoints. *Rates for American Indians/Alaska Natives are based on the contract health service delivery area [CHSDA] counties. †Hispanic is not mutually exclusive of whites, blacks, Asians/Pacific Islanders, and American Indians/Alaska Natives. Incidence data for Hispanics are based on the NHIA [North American Association of Central Cancer Registries/NAACCR Hispanic Identification Algorithm] and exclude cases from the Alaska Native Registry. Mortality data for Hispanics exclude cases from Connecticut, the District of Columbia, Maine, Maryland, Minnesota, New Hampshire, New York, North Dakota, Oklahoma, and Vermont. B, Data from SEER nine areas and U.S. mortality files [National Center for Health Statistics, CDC]. Rates are age-adjusted to the 2000 U.S. standard population [19 age groups, Census P25-1103]. Regression lines are calculated using the Joinpoint Regression Program Version 3.4.3, April 2010, National Cancer Institute [http://seer.cancer.gov/statfacts/html/lungb.html].
Surgical Pathology and Cytopathology
Adenocarcinoma
Adenocarcinomas are tumors that produce malignant-appearing glands with tubular, acinar, or papillary differentiation and whose cells may demonstrate mucin production and secretion (Box 65-1). On gross examination, the tumors may manifest peripherally with pleural retraction (puckering), as central or endobronchial masses, as diffuse pleural involvement resembling a malignant mesothelioma, arising or associated with a scar, or as a diffuse parenchymal pneumonic-like tumor. The histologic pattern and organization may show a well-differentiated acinar pattern with intracytoplasmic vacuoles or more poorly differentiated features with solid malignant growth pattern and minimal mucin expression, as demonstrated by histochemical and/or immunohistochemical staining assays. In some cases, the intracytoplasmic vacuole distends the cytoplasm and compressively deforms the nucleus to the cell margin, forming a “signet-ring” cell. The tumors may arise at various levels of the airway with malignant changes in respiratory, bronchiolar, and alveolar epithelium. Malignant transformation of these various epithelia may be associated with protean histologic and cytologic characteristics and growth patterns of invasion.
Box 65-1
Classification of Adenocarcinoma in Resected Specimens
Modified from Travis WD, Brambilla E, Noguchi M, et al: International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma, J Thorac Oncol 6:244–285, 2011.
The nature of tumor growth and progression depends on whether the cell of origin is a bronchial gland, ciliated columnar cell, goblet cell, nonciliated bronchiolar cell, or type II pneumocyte. The histologic growth pattern may show a mixture of various types including acinar, papillary, solid, and lepidic (growth along alveolar interstitium), and the cytologic features may include mucinous and nonmucinous differentiation (Figures 65-2 and 65-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


